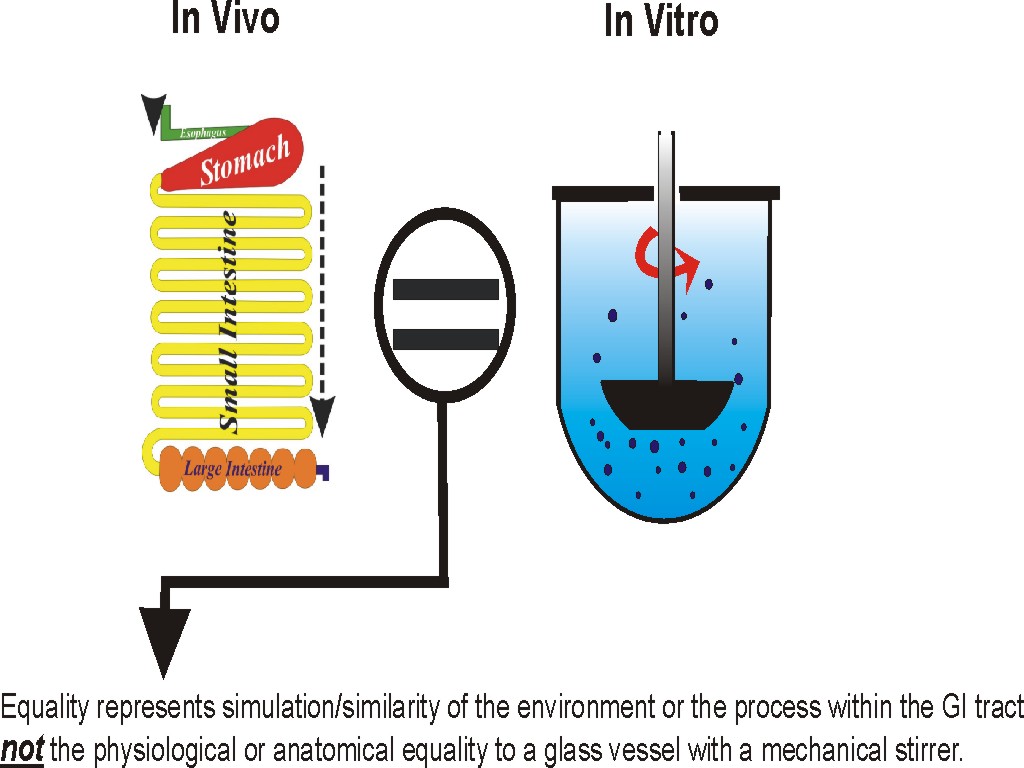
The quality control (QC) aspect of dissolution testing is linked to the release characteristics of the drug from its product, commonly tablet or capsule. This release characteristic, measured in vitro, is supposed to reflect/simulate drug release in vivo. Therefore, the QC test reflects drug release in vivo in humans, thus establishing the quality of the product. Such tests are conducted using experimental conditions that simulate human physiological conditions of GI tract as closely as possible. However, recent studies (see publication section) reflect that experimental conditions used (e.g., apparatuses) do not simulate an appropriate GI tract environment. They lack the needed mixing and stirring in the dissolution vessels. Therefore, current practices of dissolution testing may not reflect the quality of the products, and the test may not be considered a QC test.
On the other hand, considering this lack of QC aspect, commonly dissolution test is presented as a test for consistency check for batch to batch evaluations. Still, it appears to be implied as a QC test. This obviously creates significant confusion in properly describing and/or differentiating the test as a QC or consistency-check test. As stated above, in its current form dissolution test does not appear to be a QC test. Therefore, it should be considered a consistency check without its link to in vivo release and the quality of the product.
A consistency-check test may be performed using any of the experimental conditions that may or may not be physiologically relevant – for example, organic solvents vs aqueous-based, higher or lower temperatures vs 37C, any other type of stirring device (magnetic bar, shakers, propeller with high-speed motors, etc) vs commonly used paddle and basket apparatuses. Further, one may report the results for any sampling time which appear to be most stable and reproducible. This has never been the intent of the dissolution test to be conducted in this manner, particularly as a QC test.
Therefore, to conduct a dissolution test as a QC test, as was originally intended, the test must be conducted by creating or simulating a more appropriate physiological environment, i.e., improved stirring and mixing. This improved stirring and mixing aspect indeed appears to address the limitations of current practices and their artifacts. For further discussion on this topic, please see the recent literature under the publication section.