The lack of adequate stirring and mixing within Paddle and Basket apparatuses requires that the dissolution tests be conducted using product-dependent experimental conditions. For example, the experimental conditions for carbamazepine described in the USP are as follows:

As the GI tract environment remains the same for both IR and ER products, testing using the conditions mentioned above would not be physiologically relevant. Furthermore, as the experimental conditions are different for each product, one cannot make a valid comparison between products. Thus, it would be impossible to establish whether the differences in the drug release characteristics of the product are because of the products or experimental conditions
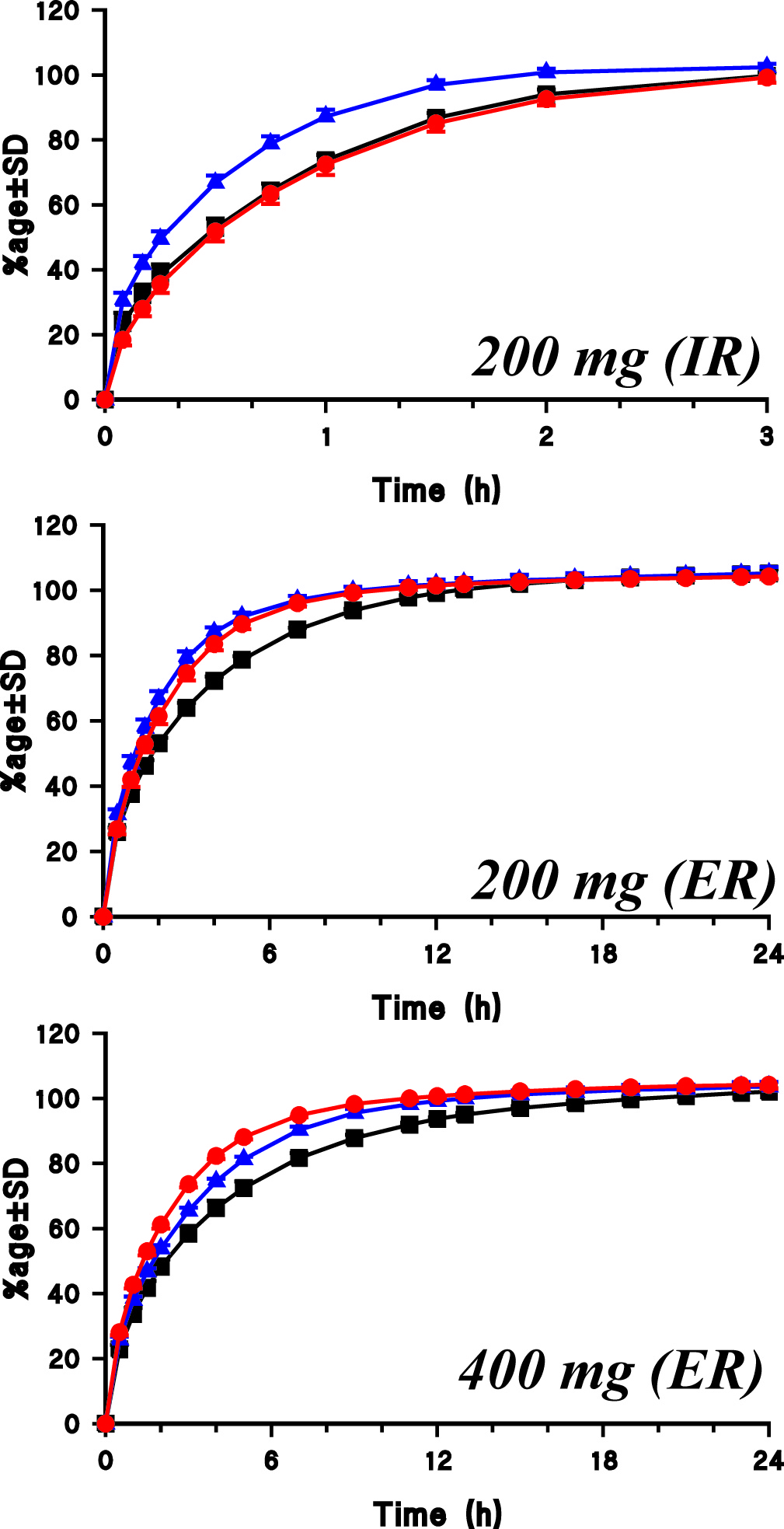
On the other hand, if appropriate stirring and mixing environments are present within the dissolution vessels, such as by using crescent-shaped spindles, then such problems do not occur. Therefore, all of the products can be analyzed using a single set of experimental conditions, such as:

The figure below shows dissolution profiles of carbamazepine products using the single set of experimental conditions as noted above (Source: Qureshi, Eur. J. Pharm. Sci., 2004).