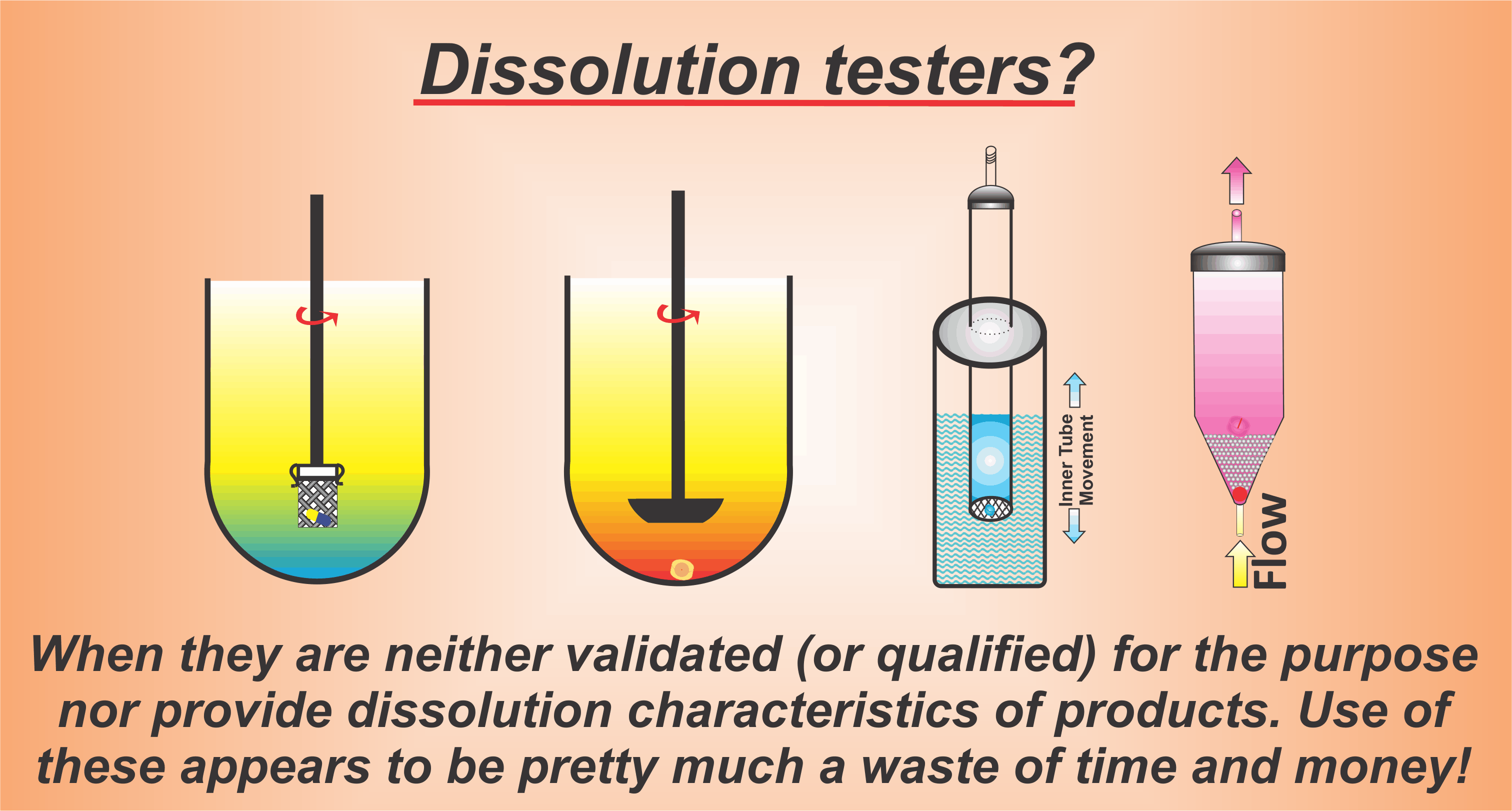
For further discussion, please see the following links: 1, 2, 3
A couple of my followers on social media brought, to my attention, a recent publication published under a variety of headings, including news media, seeking opinion concerning the validity of claims made [1, 2, 3, 4, 5].
In short, the study describes observations after inoculating the claimed SARS-COV-2 virus to healthy human volunteers to produce the virus effects.
The study claims that volunteers were “inoculated with 10 TCID50 of a wild-type virus (SARS-CoV-2/human/GBR/484861/2020) intranasally in an open-label, non-randomized study …” [2]
This statement is incorrect and deceitful.
The study did not use the virus but an “isolate.” An isolate means part/portion of the growth culture (gunk) from a swab sample, with multiple ingredients added, including cells or debris, such as Africam green monkey kidney cells (or Vero cells).
Please, follow the (link) to see the description of the “virus.” It uses the word isolate in the title, not the virus, as presumed by the study’s authors.
I previously described the differences between a virus and the “isolate” on my blog. Please see here; (1, 2).
It further confirms that there has never been a virus (SARS-COV-2) isolated, and no virus sample is available anywhere. Therefore, scientifically speaking, the virus (SARS-COV-2) and its illness (COVID-19 ) and its pandemic have been a hoax, as previously described (1,2, 3). The reported study provides further support for such an understanding.
The publication will undoubtedly cause serious damage to the scientific credibility of the journal and study authors. In addition, it will further expose the fictitiousness or fraudulent science aspect of virology.
[I posted the following comments on a discussion on the AAPS Community Forum. I think visitors to this website would also find it a helpful read].
Thanks for your comments in response to my post. I am unsure how I should respond because your comments focus more on philosophy than subject matter.
Indeed I worked with Health Canada as a research scientist to support and criticize the assignments’ underlying scientific aspects. Most of my laboratory work has been published, and views have been known publically as well – as you noted. I have never undermined any ones’, including authorities’, hard work or practices.
However, through my laboratory work and related applied experience, I did find some very disturbing misunderstandings about the use of science for the quality assessment of pharmaceutical products. Drug dissolution testing is one part of it; the second, more noticeable, is the claims of establishing the quality of the manufactured products, particularly tablets and capsules. These misunderstandings should be highlighted and addressed, in my opinion, not be ignored and concealed; otherwise, everyone involved in it will lose their credibility, in particular scientific, for a long time to come.
Your statement, “We, working in the industry, have worked very hard trying to make it work.” I am sorry, what does it mean? Can you determine the dissolution characteristics of a given blinded product sample? Have you used a validated dissolution tester for dissolution testing? How? How could you or anyone else develop a valid dissolution method without the availability of a validated dissolution tester? Can you define and establish a product’s quality using the drug dissolution method? How? Please, note that the quality of the products is not yet defined with a measurable parameter. Then how could a dissolution test be used as a quality control tool? These are some unanswered questions about using flawed science and its practice in manufacturing and regulatory assessments. These questions are not directed toward you as a person but at the industry and regulatory authorities in general. I do not know how to direct my concerns only to the regulatory authorities. I do not think that AAPS Community Forum is only for the industry. I observed it is equally read and participated in by the regulatory scientists. A discussion was just started on this community forum by a scientist from FDA (e.g., see under METHODS IN IMAGING DATA ANALYSIS).
So please join hands with me and inform the regulatory authorities that there are severe problems in regulatory requirements rather than a suggestion of avoiding discussions of these issues. This would not be public service or service to patients but something else!
You noted from my post, “A dissolution method should not be used for product development until and unless it has been clearly shown ………….” This is not my view or my suggested requirement – this is a general principle of science and regulatory (i.e., cGMP) requirement. If anyone is not following or meeting this requirement, then the results obtained would not, at least should not, be accepted under (cGMP regulation. As an example, I quote the following three FDA regulations for your information [21 CFR 111.320, 21 CFR 820.72, 21 CFR 211.194 (a) (2)]. Please correct me if I am wrong on this.
In the end, please consider using this forum to inform the authorities that fundamental scientific principles, as well as cGMP requirements, are being violated, which need to be addressed so that the industry could be able to function appropriately and the public should receive accurate and honest information about the quality of the manufactured pharmaceutical products.
I will be happy to help anyone explain the issues and suggest possible solutions to such if you suggest or provide leads in this regard. I look forward to future fruitful discussions on the subject with you.
With best regards
A discriminatory test reflects that it can differentiate a bad batch/product from a good one. The question is, how should one define bad vs. good? In general a good product, in the context of dissolution testing, is a product that should be capable of releasing a drug from the product in the GI tract in an expected and reproducible manner. It is important to note that a drug release, or dissolution test is linked to product behavior in the GI tract. On the other hand, it is a well-known fact that currently suggested dissolution methods/testers have no link to the product behavior in the GI tract (link). In fact, it is almost impossible to obtain reliable and physiologically relevant dissolution results using currently suggested apparatuses, particularly paddle/basket apparatuses, because of the flaws of the testers (link). Therefore, it is almost impossible to have discriminatory dissolution methods to differentiate a bad product from a good product by extension.
The irrelevancy of current practices of developing discriminatory tests, methods or testers may also be explained in another way with the following analogy. For example, one may ask if anyone has seen a discriminatory thermometer, laboratory balance, pH meter, spectrophotometer, chromatograph etc. The answer is, not really; because all one does, by using a tester/method, is to measure the parameter’s value. The test/method is used only to determine value/result, and the scientist/analyst uses this value for the interpretation of the characteristics of the product. For example, one never says, requires or develops a discriminatory thermometer when one monitors the temperature. One uses the thermometer to measure the value (temperature) of the corresponding parameter (body temperature). The thermometer never tells if a person has a fever or not. It only tells the temperature, but a physician interprets it as a fever or any other deviation. So, why does one have discriminatory dissolution methods or testers? It is simply to market the made-up science and flawed testers. Developing a discriminatory test/method, as practiced or required, has no meaning and serves no useful purpose. One requires a dissolution tester or method to measure the dissolution characteristics of a product reflecting it’s in vivo dissolution characteristics. Once one has such a method, it automatically becomes a discriminatory test. One does not require any extra effort or steps to make it a discriminatory test. The dissolution results thus obtained using such a method/tester would reflect dissolution characteristics of a product, and then the analyst/formulator is to decide whether the product is of acceptable quality/characteristics or not. Read the rest of this entry �
July 14th, 2013 | Author: Saeed Qureshi
The above title is self-explanatory, clear, and says it all.
When offered help in developing and/or validating dissolution methods based on non-validated apparatuses (e.g. paddle/basket) and/or experimental conditions, people have to be careful. The results obtained would not be of any use, even for QC purposes, no matter how they are presented.
The following links may be of further help in this regard
(1) If one cannot determine the dissolution characteristics of a product, then how would one be able to establish its quality or bio-relevance? A serious flaw of current practices! (link).
(2) De-aeration of a medium and vibration-free environment – perfect attention deflectors (link).
(3) Note that no one can determine, or has determined, dissolution characteristics of any product using the currently suggested apparatuses and/or methods. It has all been an illusion! (link).
(4) Assessing drug dissolution characteristics using product-dependent methods is simply unscientific and invalid practice. (link).
(5) Dissolution method development: Perhaps the most wasteful of all the current practices! (link).
(6) Current practices of drug dissolution testing using paddle/basket apparatuses – A complete waste of time! (link).
(7) Promotion of simplicity of paddle/basket apparatuses – A marketing gimmick for scientifically useless and non-validated apparatuses (link).
(8) Drug Dissolution Testing – A serious concern! (link).
(9) Drug dissolution testing: Limitations of current practices and requirements (link).
(10)Dissolution Apparatuses: Compliant vs Qualified and Validated (link).
(11)Costly mistake formulators/analysts often make, i.e., developing a product-dependent dissolution test (link).
(12)Apparatus Calibration or Performance Verification: Misleading Conclusions and False Comfort (link).
There are about 500+ dissolution methods listed in the FDA database and about 600+ methods (monographs) in the USP. In addition to these, there are many more, perhaps in the hundreds, dissolution methods described in the literature. Moreover, as part of new product development exercises, it is a common and expected practice to develop additional new or revised methods.
It may be interesting to note that the objective of drug dissolution testing has never been to develop methods but to determine/estimate drug dissolution/release characteristics of products. By developing drug and/or product-specific dissolution tests, one, in fact, would never know or determine the actual dissolution characteristics of any product. The current practices of method development simply defeat the purpose of products evaluation.
For products evaluation, one requires a test/method which is independently developed and established. Therefore, current practices of method developments are scientifically invalid and useless and a waste of time and resources.
Using the crescent shape spindle with a common set of experimental conditions is suggested to address the current difficulties. The suggested approach practically eliminates the need for method developments, particularly product dependent, and provides a scientifically sound and valid drug dissolution testing and product evaluation approach (e.g., see link, link2).
The following links may be useful for further information regarding the difficulties of the current practices:

This post is a response to a query (below). The visitors to this site may also benefit from it.
Query:
“I’ve read, albeit very little, about terrain theory. But how do you explain me and my 3 sons all catching the so called Covid and having exactly the same high temperature of 39.8? My eldest got it first in about October. Me and the other 2 at Christmas. Obviously we have shared DNA, and it looks like engineered when having the exact same temp to the decimal point in all of us.
I’m not trying to disprove you, I’d genuinely like to hear your opinion.
By the way, I’m not trying to scare monger. The high temp lasted a few hours, then 2 days of having a cold. Whereas, I had flu about 6 years ago with a high of 41.5 and a week of blah.” (link)
Response:
The common medical practice of evaluating and deciding a sickness is based on testing.
For example, one uses a thermometer to establish a higher temperature, i.e., one has a fever. Therefore, the use thermometer is a test. A thermometer is a validated tester for monitoring temperature.
The next step is to establish what may have caused this fever. There could be any number of reasons for this deviation from the norm. Usually, one assumes it is just a typical reflection of body wear and tear (called flu-like symptoms).
People generally do not consider this as sickness but a reflection of the body attempting to correct the deviation (which forms the basis of terrain theory). So bear with it or take care of it by adjusting with external help such as resting, light meals, and boosting with vitamins/fruits. I do not consider it an illness and, God forbid, a serious one.
However, if this deviation (illness) deteriorates or lingers, one has to find the reason for what may be causing it. So, one (usually a physician, like any tradesperson) guesses it based on their experience and observations and seeks its reason.
The guesses are established or confirmed by testing. There is an extensive battery of tests available. Considering my background in testing, I am not a fan of modern tests. They have weaknesses.
However, for the sake of this discussion, let us assume that these test work and provide indication and cause of illness. Once the test shows an unusual situation, one proceeds with the treatment to bring the body on a regular track.
Once the body is back in its typical form, the treatment must be stopped, especially if it is based on something the body does not need or require for its normal function, like chemical-based medicines.
Unfortunately, it has become a fashion during the past couple of years to ASSUME, immediately, any variation in normal body activity, including higher temperature, as a serious illness. This illness is named COVID-19 and is assumed to be caused by a virus attack named SARS-COV-2.
The illness must be treated immediately with the intervention of a foreign compound (or mixture) called a vaccine. Not only is the treatment suggested to bring the body to the normal stage but implied to be used continuously forever – a clear violation of living a natural and healthy life.
As stated above, illness needs to be established using a test, and this illness and its virus are confirmed based on a test called PCR. Therefore, a positive PCR test would indicate COVID-19.
However, the biggest problem with the test is that it is scientifically irrelevant and invalid. There should not be any doubt or question about it. The reason is that for a test to be relevant and valid, it must be validated against the substance it is supposed to test, in this case, the virus (SARS-COV-2). However, it has not been because no virus specimen is available to develop and validate the test.
Therefore, people should recognize that there is no such thing as the virus (SARS-COV-2, variants, etc.) of its illness (COVID-19).
Considering your specific situation, you all recovered from your illness fairly quickly and without any serious impact. This would support the assumption that your body was going through normal wear-tear, nothing more. So, enjoy your life and health without unnecessary worries.
Concerning your link with children through DNA and the similarity of illness, this is just a fancy talk “experts” tell you to sell you the products and expertise. Scientifically, the link of DNA to illness is in its infancy stage (if it is there at all). Working as a scientist in this area, I have difficulty believing and accepting its accuracy and relevance. At this stage, it is a marketing tool to impress people and promote products and services, which are, otherwise, a waste of money and good health.
I am unsure if this explanation will help you and your children escape the fear of illness or bad health. However, it is worth the try.
For further information, please consider obtaining a copy of Helpful Notes, where I describe scientific aspects of modern-day medicines in simple language.
I wish you the best, and be vigilant of modern-day health experts and their advice.