As a part of a LinkedIn discussion (link), I provided the following (below) response to a query. The visitors of this blog may find my response informative and useful as well.
Steve:
Thanks for your kind words about my article and also for asking the questions.
First, to be clear that I am not a microbiologist or virologist. I am a chemist and have worked in the pharmaceuticals area for 30+ years (as a scientist with Health Canada). I gained significant experience and expertise in critically evaluating pharmaceutical products.
Regarding the claim of virus isolation, I am saying that the experiments microbiologists/virologists perform and describe, such as virus characterization, identification, belongs to the chemistry discipline. However, the chemistry work has not been conducted accurately; hence claims made are incorrect.
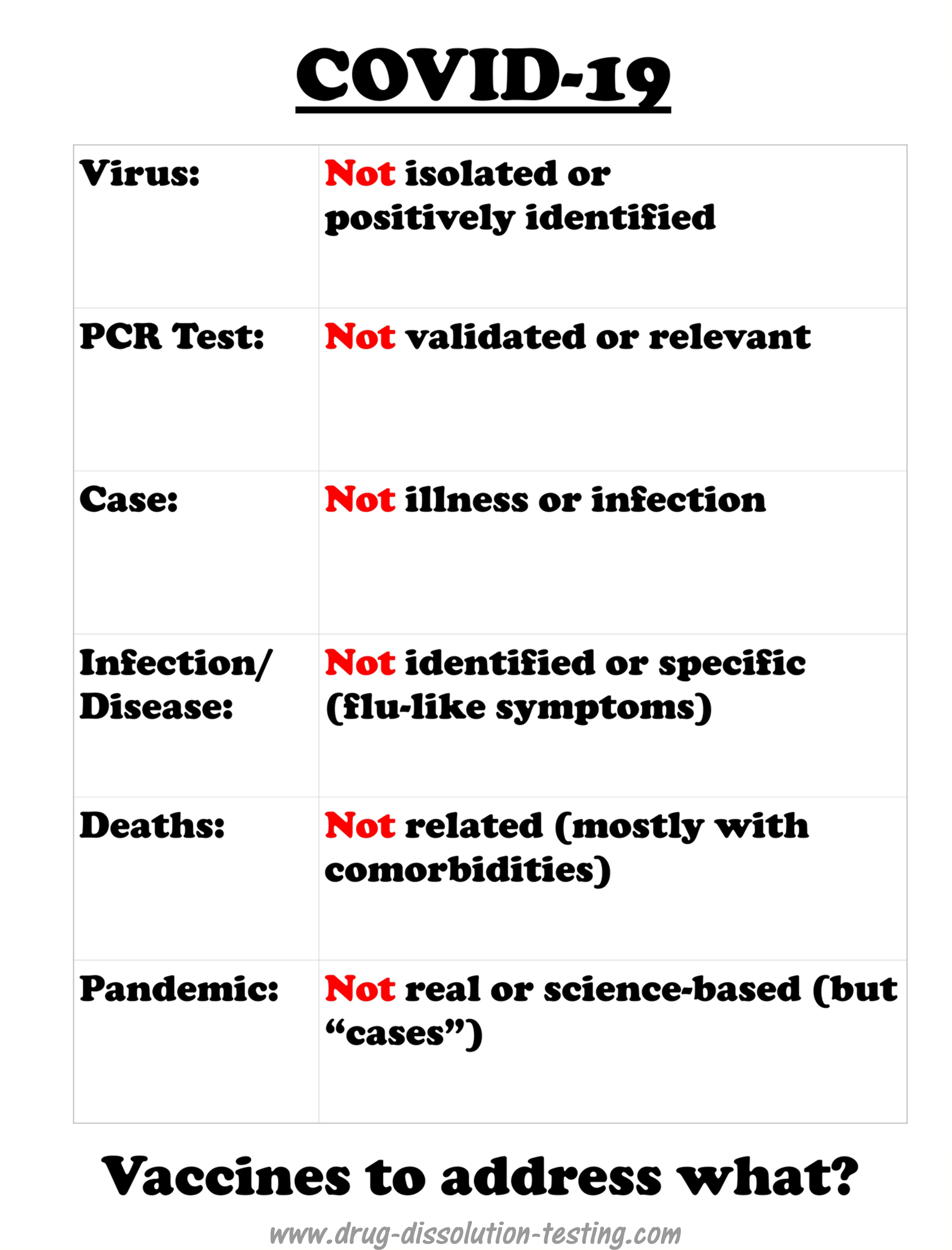
There are many ways to describe and explain the inaccuracies. One of which is that of isolation of a substance, in this case, a virus. If one needs to isolate a virus, one must go through multiple steps, such as extraction, purification, identification, and structure determination, resulting in a pure sample of the virus. Nothing of this sort has been done for the virus isolation, in particular, SARS-CoV-2. Therefore, it cannot be said that the virus has been isolated and identified, or even it exists.
On the other hand, microbiologists and virologists (among others) work with a modified definition and description of the term isolation, for their purpose, as taking a swab sample (i.e., separating virus from the host). That is not a good scientific practice. Therefore, in reality, a virus never gets isolated in its true or pure form.
On the other hand, to appear scientific, the DNA/RNA sequencing is considered as a claim of “identifying” the virus in a swab sample without truly “isolating” it (we chemists call it a clean-up step). Some chemical steps using enzymes (commonly known as polymerization, again a chemistry step) are conducted, followed by taking some pictures of the soup with an electron microscope. Observing spherical bodies with spikes is considered to reflect the existence of coronavirus.
From this soup (note everything is from the soup, nothing from pure virus), DNA or RNA or its fragments are extracted to establish their sequences. There is no evidence that this DNA or RNA is from anything specific, including the virus – it is an assumption. Based on computer analysis and comparison with previously obtained “reference” sequence (usually obtained from WHO depository), which is also “isolated” in a similar manner (without isolating the virus), virus existence is established. If the sequence did not match the “reference” sequence (not a virus), it would become a new virus or new strain.
The publications, links you provided all follow the same or similar protocol as I summarized above. I have critically reviewed two such publications, one from Australia (the one you noted in your post as well), the other from the USA (CDC), where I explained: “science” behind “isolation” of the virus. Links are provided (link1, link2) please have a look.
I have been arguing for some time that nowhere I can find an isolated virus, so why do people keep claiming isolated virus. My recent discussion with a microbiologist made it clear that the virus has never been isolated but misrepresented by incorrect definition of the word “isolation.” That makes it clear why I could never find the sample and specimen of the pure virus because it does not exist. One may imagine my shock after hearing this – so I wrote the article.
I hope I answered your query adequately. Otherwise, let me know. I will explain it further. I like to make another point, without going into technical details, the sequencing (chemistry) part is pretty iffy. It is well known to the people in the area that sequencing steps can produce highly unpredictable results. The PCR test, which in reality is based on sequencing, suffers this weakness. Hence one sees so many false positive or negative outcomes that make the lack of “virus existence” claim even stronger. (edited)