The quality of pharmaceutical products such as tablets/capsules may be defined as their ability to provide or release the drug present in the product in humans in an expected manner. It is important to note that a consumer or patient needs to take a drug product to have the drug (active ingredient) delivered to them in the right amount and in an expected manner. Therefore, a product provides only the means to deliver this drug.
For example, as shown above, a consumer requires juice, but the carton provides a means to deliver the juice to the consumer. If the juice comes out of the carton as expected, then the product becomes of quality. However, if the carton is unable to pour out (deliver) the juice in the expected amount and in a consistent manner, even if the juice is present inside the carton, the product would not be considered of quality. The point is, even if the drug in a tablet/capsule is of the highest quality, but the product (tablet/capsule) is unable to release it, the product will be considered a poor quality product.
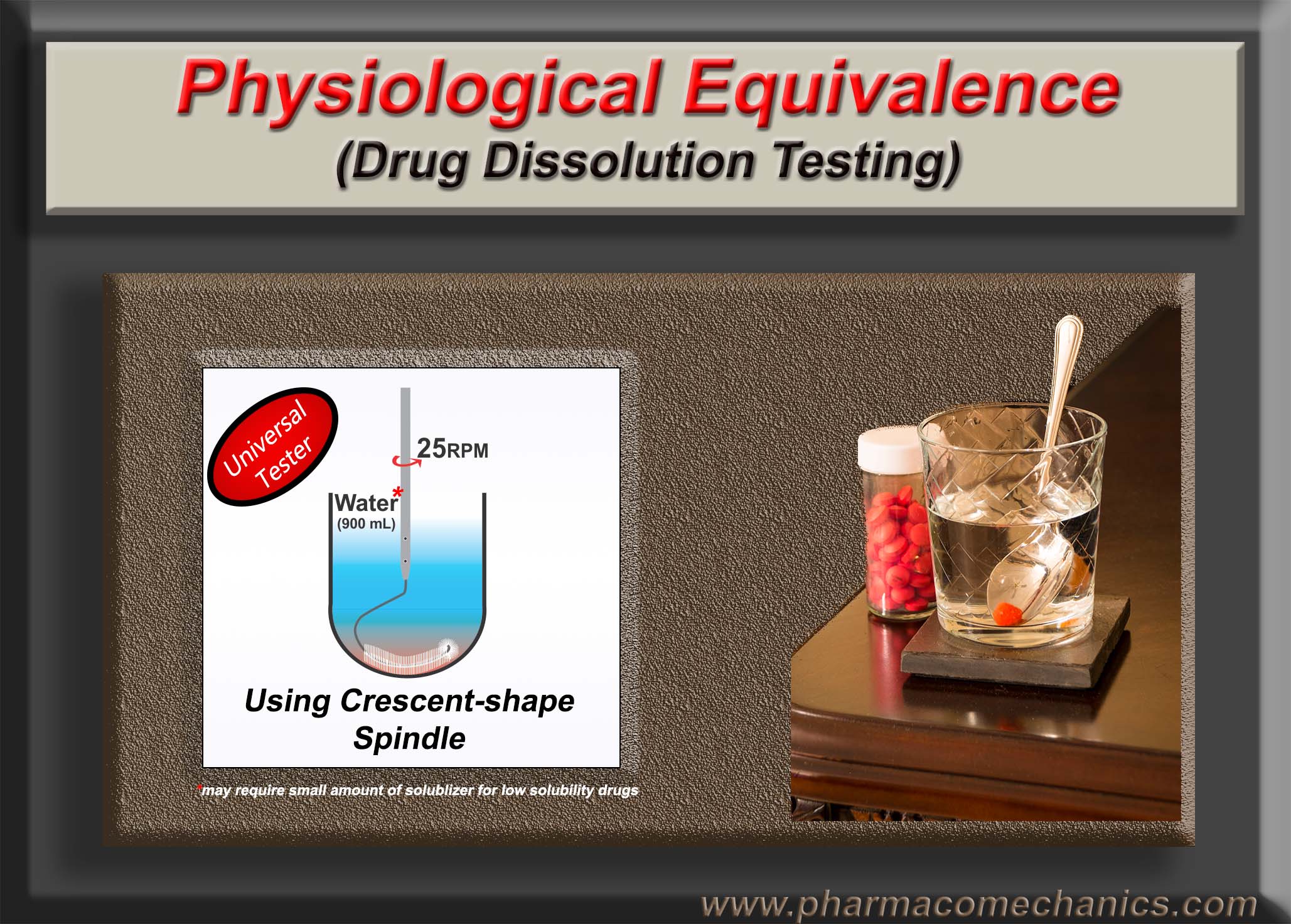
To assess the release characteristics of the drug products, thus quality, commonly a pharmacopeial test known as drug dissolution test is conducted – a worldwide regulatory requirement for the approval and marketing of products. The most commonly used standards or specifications for such purposes are pharmacopeial (e.g., USP), known as Q-based Acceptance Criteria. The Criteria may be summarized as follows: if 24 units (capsules/tablets) are tested in a sequence of 6, 6, and 12 units, and the results are such that, on average, units provide drug release of not less than 80% (Q-value of the product) of the labeled amount, while two units may release between 65% to 80% and one of the units may have a drug release of 55%. The product meeting these criteria will be considered approved or of quality. As the percentage of drug release is Q dependent, if the Q-value is lower for a product, the corresponding expected drug release would be lower.
To explain these complex approval criteria in simple terms, with the example of a 2L carton of juice, it would mean that juice cartons will be considered as a quality product if: they have a Q-value of (80%), for 24 cartons, consumers should expect on average 1.6L (80% of 2L) of juice be poured out, and from two cartons it may be between 1.3 to 1.6L (65-80%) and for one carton 1.1L (or 55%). It should be obvious that if such standards/specifications are to be followed by carton manufacturers, which are equivalent to drug product manufacturers in this case, they would be out of business immediately for providing such extremely poor quality products. However, the pharmaceutical industry follows the such standard, as a norm, with claims that they follow stringent quality standards. So how does it make sense that a consumer/patient is expected to receive at random 55% to 80% of the labeled drug amount, and products are considered quality?
Therefore, it is humbly requested that regulatory authorities take note of such bizarre and poor criteria for establishing the quality of pharmaceutical products. Please pay attention!