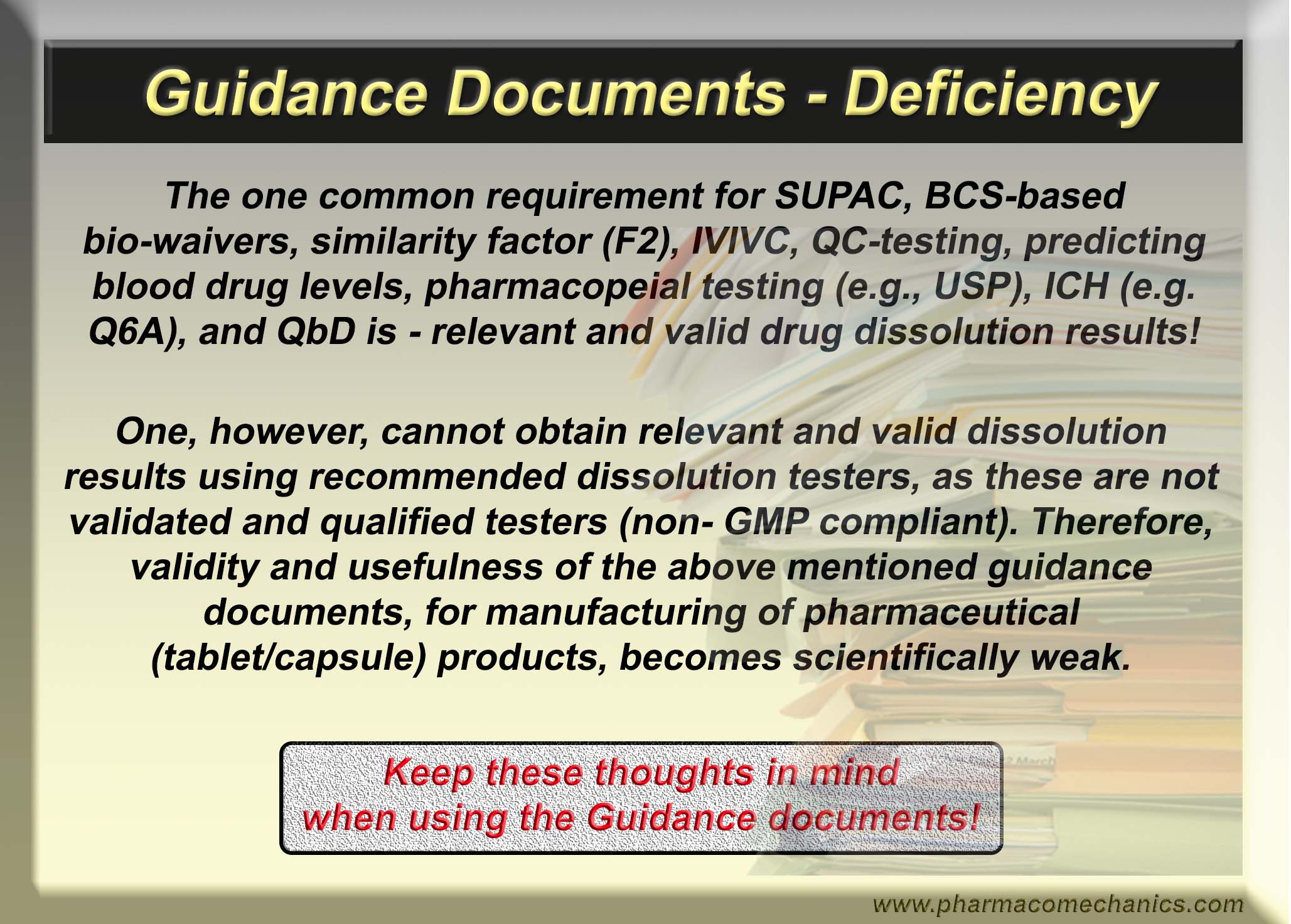
Dear regulatory and pharmaceutical scientists, please note that all dissolution tests described in the USP and/or FDA database lack scientific basis and are invalid for assessing any drug product characteristics, including quality. The recommended testers and associated methods have never been validated and/or qualified for the intended purpose. These tests are conducted as a regulatory requirement, which forces people/industry to use the testers and the methods resulting in false claims regarding the quality of products. To be scientifically valid, the tests must be product-independent, and testers must be validated and qualified for testing products for human use. Please pay attention!
Author: Dr. Saeed Qureshi, Ph.D.
The tester must be shown first that it is capable of providing relevant dissolution results of a product with low agitation (stirring) environment i.e. it is capable of providing expected and consistent dissolution results with acceptable variability e.g. 10 % CV or RSD. (Physical or mechanical requirement)
The dissolution results obtained must relate to the physiological release characteristics of the product using a physiologically simulated medium (solvent) i.e. a fast drug-release product to provide faster dissolution results while slow drug-release to provide slower drug dissolution results without changing the experimental/testing conditions i.e. test/tester must be product independent. (Physiological requirement)
Since, none of the currently recommended testers and/or methods, including pharmacopeial and/or regulatory, meets the above mentioned requirements; they cannot be considered as bio- or clinically relevant testers or methods. In addition, since none of the currently recommended testers have been shown to meet condition #1 (above), these cannot be considered capable of providing bio- or clinically relevant tests/methods. Suggesting otherwise would be a scientifically invalid practice or claim.
For further discussion on the topic please follow the link.