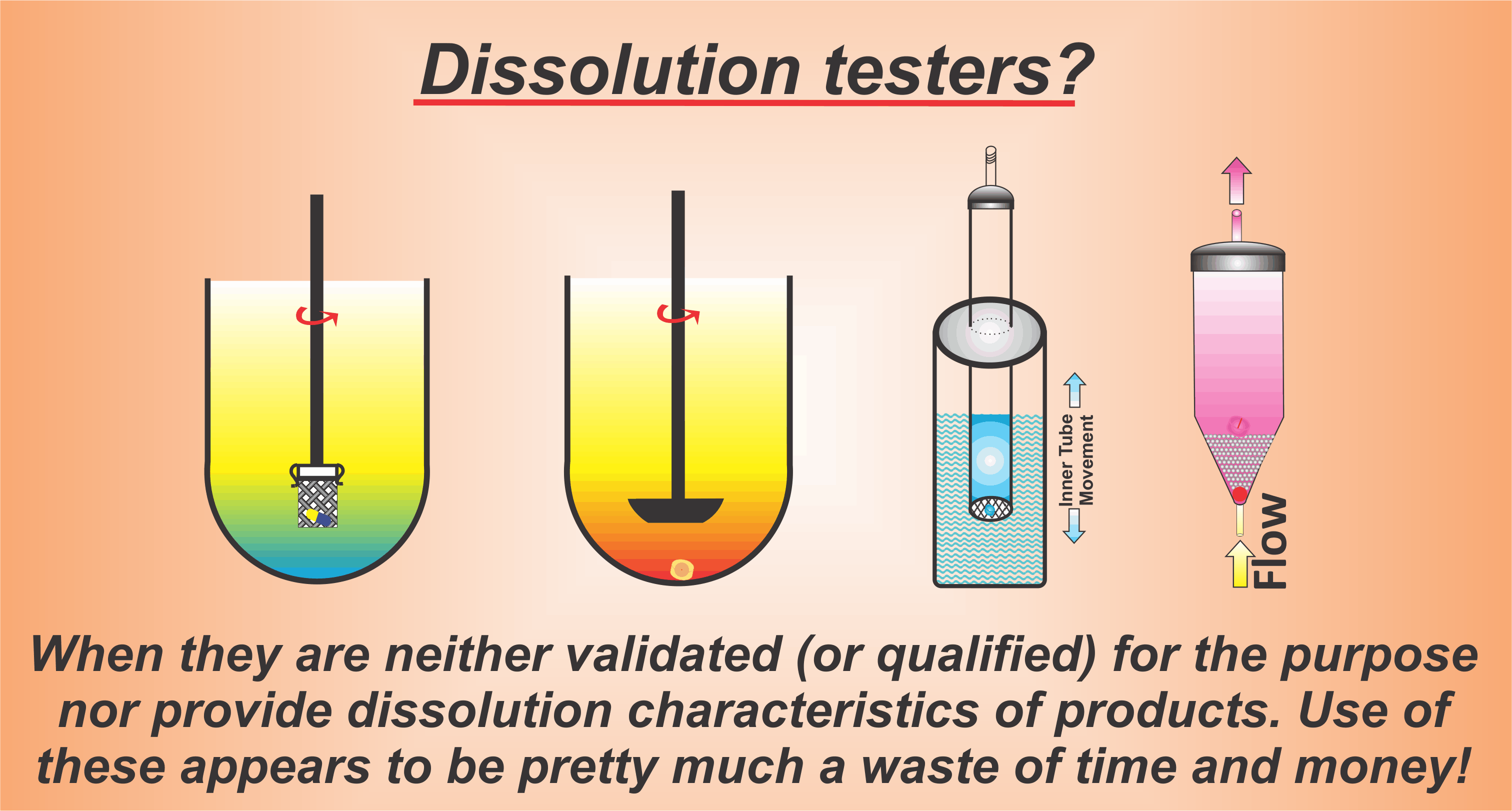
For further discussion, please see the following links: 1, 2, 3
While surfing the net, I came across a response to a query published in Dissolution Technologies (see issue of February 2011 Volume 18 Issue 1, Question & Answer Section) regarding product-specific dissolution testing. It is quite disturbing to read that such poor and irrational scientific reasoning can be provided, for multiple and product-dependent, dissolution tests in the pharmacopeia. The suggested reasons are scientifically invalid and provide a strong case for removing the tests and standards from the compendia, which by definition is expected to set unbiased and independent product standards. For example:
It is stated that “We may find tempting the notion that because products may have similar doses and dosing intervals, they should have the same dissolution test. In the present state of the art, that is simply not the case for extended-release products.” On the other hand, such a practice is valid for immediate-release (IR) products because the same dissolution tests are recommended for IR products (e.g., generics) having similar doses and dosing intervals. It is unclear how a dissolution tester and/or test will differentiate between IR and extended-release (ER) products and will start behaving differently by providing unacceptable results for ER products only.
It is stated that “While bioequivalence is used to establish generic status, the release mechanisms of reference listed drugs and generics may not produce adequately similar profiles in the dissolution test conditions to satisfy one quality control performance test.” A “generic status” means that drug release characteristics of the reference and generic products are the same. The composition, manufacturing and/or mechanism attributes of the reference and generic products are always different. That is why bioavailability/bioequivalence studies are required and conducted to establish that given the differences in products, their drug release characteristics MUST be the same. So, if products are bio-equivalent, i.e., having the same drug dissolution/release profiles in vivo, and another test, in vivo or in vitro, shows differences in drug release, how relevant or useful would such a test be? The responder, in fact, appears to be arguing for developing an irrelevant dissolution test, for ER products, which would show differences in vitro dissolution/release profiles for products having the same or similar in vivo profiles.
It is further stated that “Depending on your use of the dissolution test, you may need to develop a test specifically for the product in hand.” What should be the basis (or criteria) for developing the test for products having similar in vivo dissolution profiles if that is the case? If it is accepted that dissolution tests are to be developed as product dependent and not necessarily linked to in vivo characteristics of the product, then a test having the attributes of 80% drug dissolved in 45 minutes using water with 50% alcohol as the dissolution medium with the paddle (250 rpm) has to be considered satisfactory. Otherwise, current pharmacopeial drug dissolution testing requirements may be considered based on the principle of “follow the instructions” and not science and/or logical reasoning.
It appears that the responder has provided a strong case for the discontinuation of the pharmacopeial dissolution standards and/or requirements, at least for the ER products. This may be explained as follows: (1) if the tests and tolerances are linked to a specific product as suggested, then such standards may become proprietary and may be difficult for others to meet and/or duplicate; (2) if the reported tests or standards in the pharmacopeia are not required or expected to be met then why are these in the pharmacopeia; (3) in the absence of such requirements, obviously one is free to develop any tests and/or tolerances, with or without the relevance to in vivo drug release. The question is, then again, what is the use of setting public standards?
In short, it may be argued that the current practices of setting multiple and product-dependent dissolution tests are scientifically invalid and provide no useful purpose; thus should be discontinued.
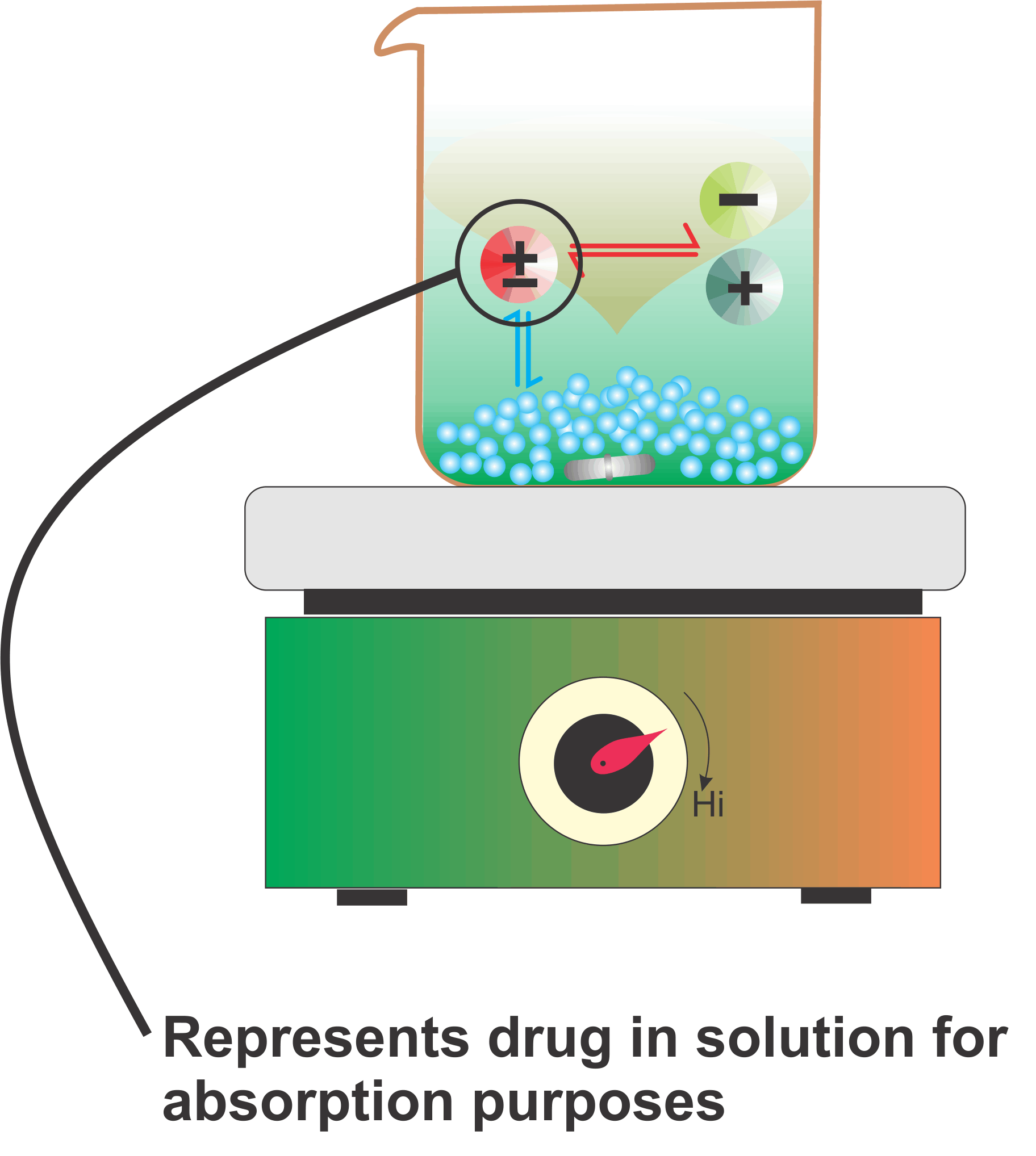
The Figure is a simple schematic representation for explaining the solubility of drugs for absorption purposes in the human GI tract for drugs that are weak acids or bases. These drugs dissociate into ions in equilibrium with the undissociated molecules (drugs) in the solution.
The undissociated drugs get absorbed, which disturbs the equilibrium with the corresponding ions. To maintain the equilibrium, the drug moves from the solid to the dissolved (undissociated) form, which gets extracted/absorbed. This cycle continues until the entire drug gets absorbed. The important thing to note here is that for complete absorption, drugs are neither required to be highly soluble nor need a large volume of solvent. It is this continuous extraction/absorption step that makes drug absorption possible and efficient.
For further discussion on the topic, please see the following links: (1, 2, 3, 4)
Dissolution tests are conducted for solid oral products such as tablets/capsules to simulate/evaluate in vivo drug dissolution required for the absorption of drugs from the GI tract to exert their therapeutic effects. Therefore, for appropriate absorption, drugs should dissolve in the liquid present in the GI tract. The liquid present in the GI tract is simulated in vitro with water or aqueous buffers having a pH in the range of 1 to 7.
Commonly in literature, three pH values are suggested, which are 1, 4.5, and 6.8 to cover the range of pH of the GI tract. It is possible, in fact quite common, that a drug may be freely soluble at one pH but not the other. For example, acidic drugs such as NSAIDs (e.g., ibuprofen) would practically be insoluble in a solution having a pH of 1 but will be freely soluble at pH 7. So, how should one decide, for dissolution testing purposes, whether such drugs are of high or low solubility characteristics, and how should they be tested? Please click here for the complete article
To avoid potential frustrations and unnecessary workloads, when conducting dissolution tests one should be watchful of the following limitations of the currently suggested practices and requirements (please follow the links provided within brackets for further details on the topic).
For assessing the potential absorption behavior of drugs from the GI tract, the following points may be helpful:
The following links are for the short video clips demonstrating comparative operations of the paddle and the crescent-shaped spindles.
Using a disintegrating tablet: Paddle and Crescent-shape spindle
Using a non-disintegrating tablet: Paddle and Crescent-shape spindle
Note: Dimensions may appear slightly distorted
In an earlier article (link) the mechanism of drug absorption was described considering ionization characteristics of drugs and the differences between the surface areas of the stomach and intestine. The purpose of the article was to explain and highlight how ionization of both an acidic and basic drug will provide undissociated drug molecules both in the stomach and intestine. It is important to note that the undissociated drug molecules in the solution form are responsible and required for drug absorption.
The low (acidic) pH of the stomach would favor high undissociated concentrations of acidic drugs compared to high (neutral or basic) pH of the intestine, which will favor higher dissociated (ionized) concentrations. The opposite is true for basic drugs where the stomach’s low (acidic) pH will result in higher concentrations of ionized or protonated basic drugs in the stomach compared to higher concentrations of undissociated drugs in the intestine. Thus, the pH of the environments (stomach and intestine) explains only the ionization of drugs (acidic or basic), i.e., comparative availability of undissociated drug molecules in solution form but NOT the EXPECTED absorption of the drugs from these sites. However, the absorption of drugs can only be explained based on the available surface areas of the stomach and intestine. As the intestine provides a much larger and efficient (permeable) surface compared to the stomach, thus it provides far superior and efficient drug absorption, as explained in the previous article.
It is sometimes assumed that as acidic drugs are more readily available in the undissociated form in the stomach (low pH), they will preferentially be absorbed from the stomach. The basic drugs will be more readily available in the undissociated form in the intestine (neutral or higher pH) thus will preferentially be absorbed from the intestine. This assumption is inaccurate, as such a simplistic approach ignores the absorption capacity and differences of surface areas of the stomach and intestine sites. Furthermore, such an assumption i.e. absorption based on pH consideration only contradicts the observations reported extensively in the literature. For example:
An important implication of such a physiological process (i.e. drugs absorption from the intestine) is that a dissolution test should also be conducted simulating the intestinal environment, e.g., using a dissolution medium having a pH in the range of 5 to 7. Conducting the tests using acidic pH (simulating the stomach environment) may not be appropriate when the dissolution results are related to physiological outcomes such as plasma drug levels.
This article provides an overview of the mechanism of drug absorption from the GI tract based on solubility/dissolution and dissociation/pH characteristics of a drug. It is argued that although pH values of the environment (stomach and intestine) may play a role, it is the availability of the large surface area of the intestine which predominantly is responsible for the drug absorption for both acidic and basic drugs. Furthermore, in the GI tract, drugs exist in three forms, i.e., solid (outside solvent/solution) and solid and ions in solution which are in equilibrium with one another. However, it is only the drug in a solution form which is relevant for the absorption purpose. The interactions between drug (solid), drug/ions in solution, and the surface areas are discussed in providing efficient drug absorption. Considering the absorption mechanism, the role of in vitro drug dissolution testing is also highlighted. Please click here for the complete article
The similarity Factor or F2 is a parameter commonly used to show the similarity or equivalence of two dissolution profiles. The F2-value is often calculated using the formula described here (link)—the values of F2 range from 0 to 100. Commonly, a value between 50 and 100 is considered to reflect the similarity of two dissolution profiles, which implies that the products will have similar in vivo drug release characteristics (please click here for complete post).