Last month I gave a seminar in Lahore, Pakistan, organized by the Pakistan Pharmaceutical Manufacturers’ Association (PPMA). A copy of the presentation (slides) is attached (link). If you have any comments and/or questions, please do not hesitate to contact/write me at moderator@drug-dissolution-testing.com
There are a number of reasons why one can use, in fact, should use, non-compendial testers and methods, e.g., when currently recommended compendial testers/methods are:
- Unable to determine dissolution characteristics of a blinded sample, a common and standard practice/requirement for any analytical test.
- The usefulness (validity) of the tester/method is in question, i.e., a tester/method is not capable of determining and differentiating dissolution characteristics of products. For example, they cannot differentiate between immediate- and extended-release products having the same active ingredient.
- The underlying science is weak or invalid, e.g., the test method is product-dependent. It is a severe scientific violation that the method used is product-dependent.
- Complex and time-consuming.
Pharmacopeial testers/methods are deficient in all the above-mentioned characteristics/requirements. Therefore, if a newer approach addresses any or all of the above-mentioned deficiencies, then automatically, the new or modified tester/method becomes superior and should be used. Therefore, people should focus on addressing and improving current methods and proactively suggest newer methods to the agencies. Analysts/scientists should not consider views that only pharmacopeial testers/methods should be employed to avoid potential delays in product approval. Spending some extra time upfront to have a scientifically valid and robust method is much better. Rather than using the recommended method/approach, which is flawed as it will cost a lot more during production cycles, failures (OOS), recall, etc.
It is important to note that modified testers/methods should be suggested at the product application stage, where it would be relatively easier to accept. It is highly unlikely to change at the production or plant inspection stage, as specifications are part of the “contract,” which are very difficult or impossible to change.
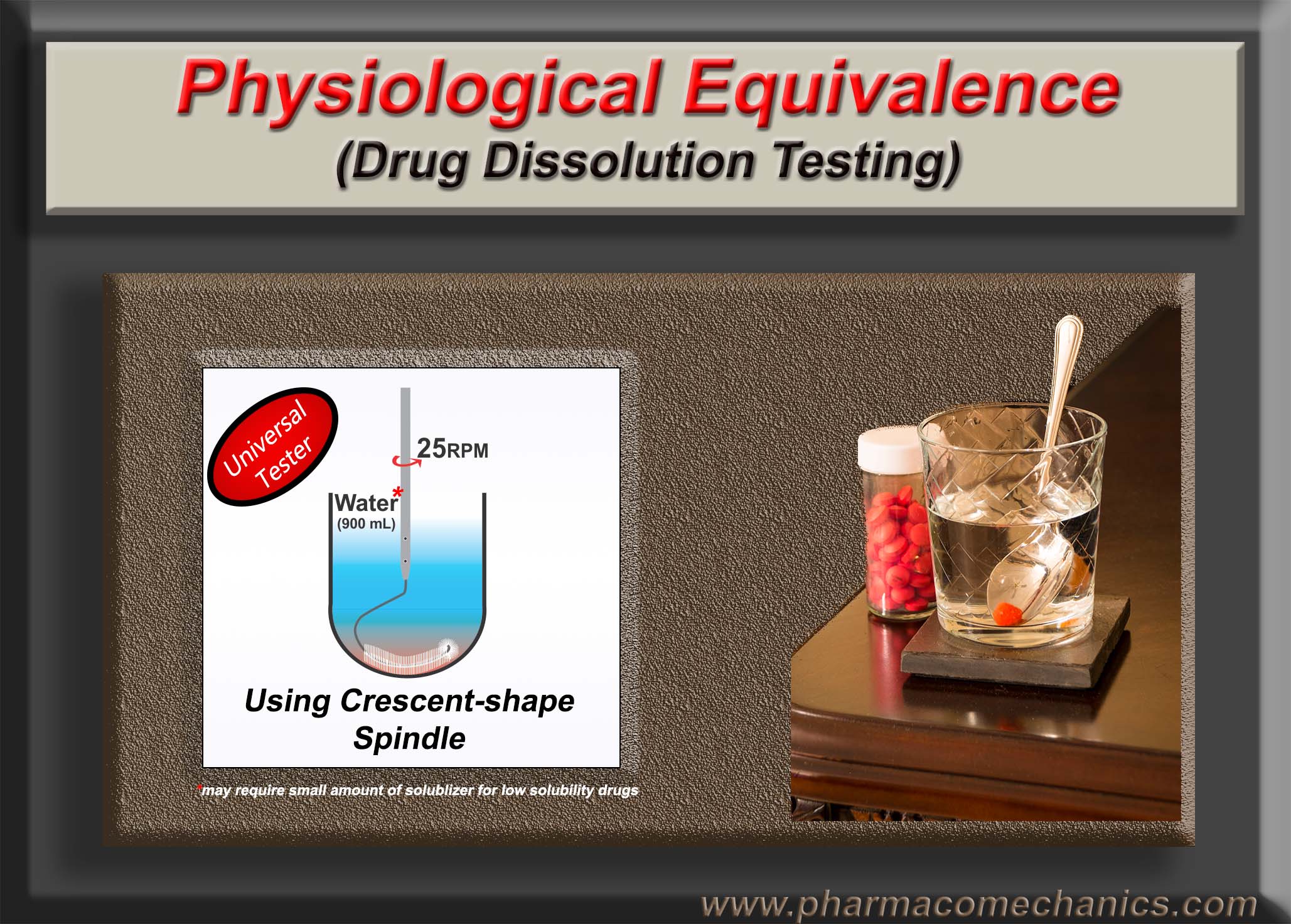
The Crescent-shaped spindle (link) has been suggested for drug dissolution testing to address the deficiencies of the currently recommended apparatuses and methods. In addition, its use significantly reduces the workload, thus increasing efficiency and cost savings.

The quality of pharmaceutical products such as tablets/capsules may be defined as their ability to provide or release the drug present in the product in humans in an expected manner. It is important to note that a consumer or patient needs to take a drug product to have the drug (active ingredient) delivered to them in the right amount and in an expected manner. Therefore, a product provides only the means to deliver this drug.
For example, as shown above, a consumer requires juice, but the carton provides a means to deliver the juice to the consumer. If the juice comes out of the carton as expected, then the product becomes of quality. However, if the carton is unable to pour out (deliver) the juice in the expected amount and in a consistent manner, even if the juice is present inside the carton, the product would not be considered of quality. The point is, even if the drug in a tablet/capsule is of the highest quality, but the product (tablet/capsule) is unable to release it, the product will be considered a poor quality product.
To assess the release characteristics of the drug products, thus quality, commonly a pharmacopeial test known as drug dissolution test is conducted – a worldwide regulatory requirement for the approval and marketing of products. The most commonly used standards or specifications for such purposes are pharmacopeial (e.g., USP), known as Q-based Acceptance Criteria. The Criteria may be summarized as follows: if 24 units (capsules/tablets) are tested in a sequence of 6, 6, and 12 units, and the results are such that, on average, units provide drug release of not less than 80% (Q-value of the product) of the labeled amount, while two units may release between 65% to 80% and one of the units may have a drug release of 55%. The product meeting these criteria will be considered approved or of quality. As the percentage of drug release is Q dependent, if the Q-value is lower for a product, the corresponding expected drug release would be lower.
To explain these complex approval criteria in simple terms, with the example of a 2L carton of juice, it would mean that juice cartons will be considered as a quality product if: they have a Q-value of (80%), for 24 cartons, consumers should expect on average 1.6L (80% of 2L) of juice be poured out, and from two cartons it may be between 1.3 to 1.6L (65-80%) and for one carton 1.1L (or 55%). It should be obvious that if such standards/specifications are to be followed by carton manufacturers, which are equivalent to drug product manufacturers in this case, they would be out of business immediately for providing such extremely poor quality products. However, the pharmaceutical industry follows the such standard, as a norm, with claims that they follow stringent quality standards. So how does it make sense that a consumer/patient is expected to receive at random 55% to 80% of the labeled drug amount, and products are considered quality?
Therefore, it is humbly requested that regulatory authorities take note of such bizarre and poor criteria for establishing the quality of pharmaceutical products. Please pay attention!

The quality of pharmaceutical products such as tablets/capsules may be defined as their ability to provide or release the drug present in the product in humans in an expected manner. It is important to note that a consumer or patient needs to take a drug product to have the drug (active ingredient) delivered to him/her in the right amount and in an expected manner. Therefore, a product provides only the means to deliver this drug.
For example, as shown above, a consumer requires juice, but the carton provides a means to deliver the juice to the consumer. If the juice comes out of the carton as expected, then the product becomes of quality. However, if the carton cannot pour out (deliver) the juice in the expected amount and in a consistent manner, even if the juice is present inside the carton, the product would not be considered of quality. The point is, even if the drug in a tablet/capsule is of the highest quality, but the product (tablet/capsule) is unable to release it, the product will be considered a poor quality product.
To assess the release characteristics of the drug products, thus quality, a pharmacopeial test known as drug dissolution test is conducted – a worldwide regulatory requirement for the approval and marketing of products. The most commonly used standards or specifications for such purposes are pharmacopeial (e.g. USP), known as Q-based Acceptance Criteria. The Criteria may be summarized as if 24 units (capsules/tablets) are tested in a sequence of 6, 6, and 12 units, and the results are such that, on average units provide drug release of not less than 80% (Q-value of the product) of the labeled amount, while two units may release between 65% to 80% and one of the units may have a drug release of 55%. The product meeting these criteria will be considered approved or of quality. As the percentage of drug release is Q dependent, if the Q-value is lower for a product, the corresponding expected drug release would be lower.
To explain these complex approval criteria in simple terms, with the example of a 2L carton of juice, it would mean that juice cartons will be considered as a quality product if: they have a Q-value of (80%), out of 24 cartons, consumers should expect on average 1.6L (80% of 2L) of juice be poured out, and from two cartons it may be between 1.3 to 1.6L (65-80%) and for one carton 1.1L (or 55%). It should be obvious that if such standards/specifications are to be followed by carton manufacturers, which are equivalent to drug product manufacturers in this case, they would be out of business immediately for providing such extremely poor quality products. However, the pharmaceutical industry follows such standards, as a norm, with claims that they follow stringent quality standards. How does it make sense that a consumer/patient is expected to receive at random 55% to 80% of the labeled drug amount, and products are considered as of quality?
Therefore, it is humbly requested that regulatory authorities take note of such bizarre and poor criteria for establishing the quality of pharmaceutical products. Please pay attention!

Drug dissolution testing is a well-established analytical technique or test and is extensively employed for the development and assessment of pharmaceutical products, in particular, tablets and capsules. It would not be an exaggeration to say that it is the only test used to establish the quality of products. However, the tests and testers have never been qualified and validated for their intended purpose. Therefore, tests and testers cannot provide relevant and scientifically valid results regarding the quality of the tested products. This is simply common sense and scientific fact.
Some, however, still promote the current practices through training (conferences, seminars, and write-ups) as scientific and useful, which are not only causing delays and hindrances in addressing the issues at hand. This also creates false hope for analysts/scientists that this technique may provide useful and relevant results. Sometimes this training and advice are disguised with different fanciful names or topics such as clinical or bio-relevant, IVIVC, and bioavailability/bioequivalence assessment. Still, underneath it, all are flawed dissolution results. It is impossible to obtain reproducible and relevant dissolution results for any product using the currently suggested dissolution testers.
A simple and practical approach one could use to assess the credibility or authenticity of promoted claims and expertise is to request if the person (trainer or vendor’s representative) can determine dissolution characteristics of a given blinded sample of a product containing a highly soluble drug using an independently developed and validated method (see below). If yes, it could certainly be a good source for learning. Otherwise, one should use caution.
Please use caution and pay attention (link)