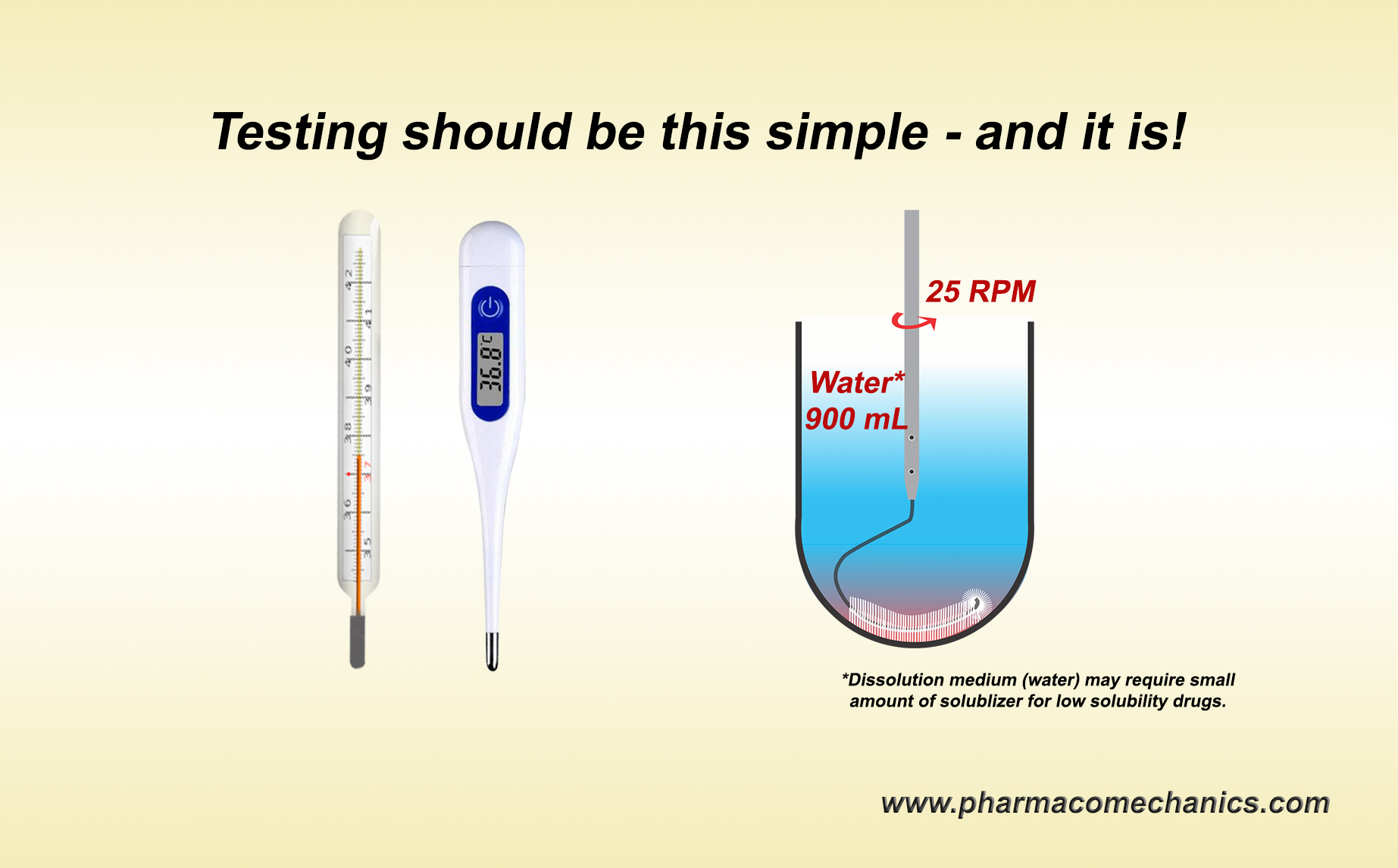
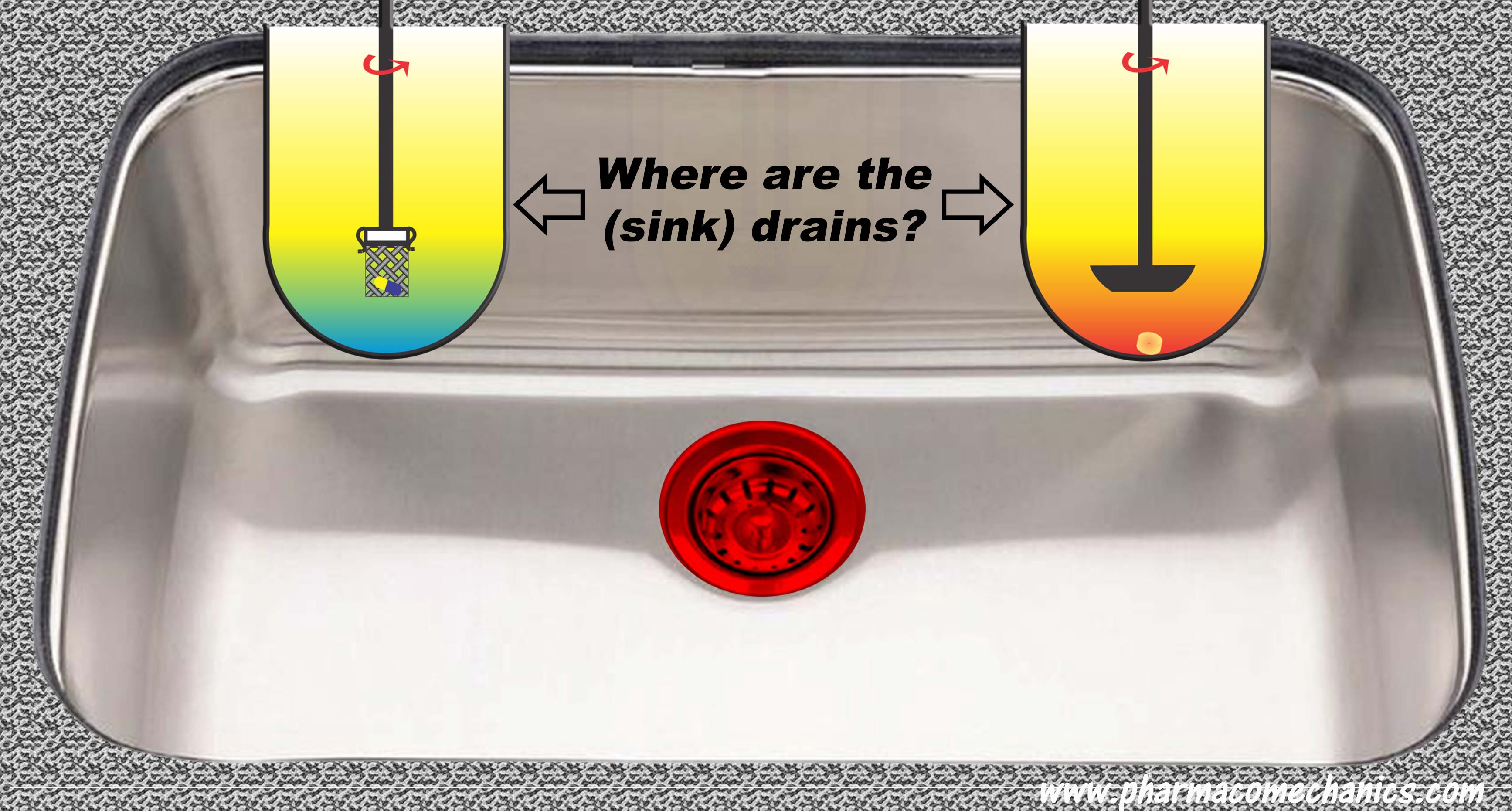
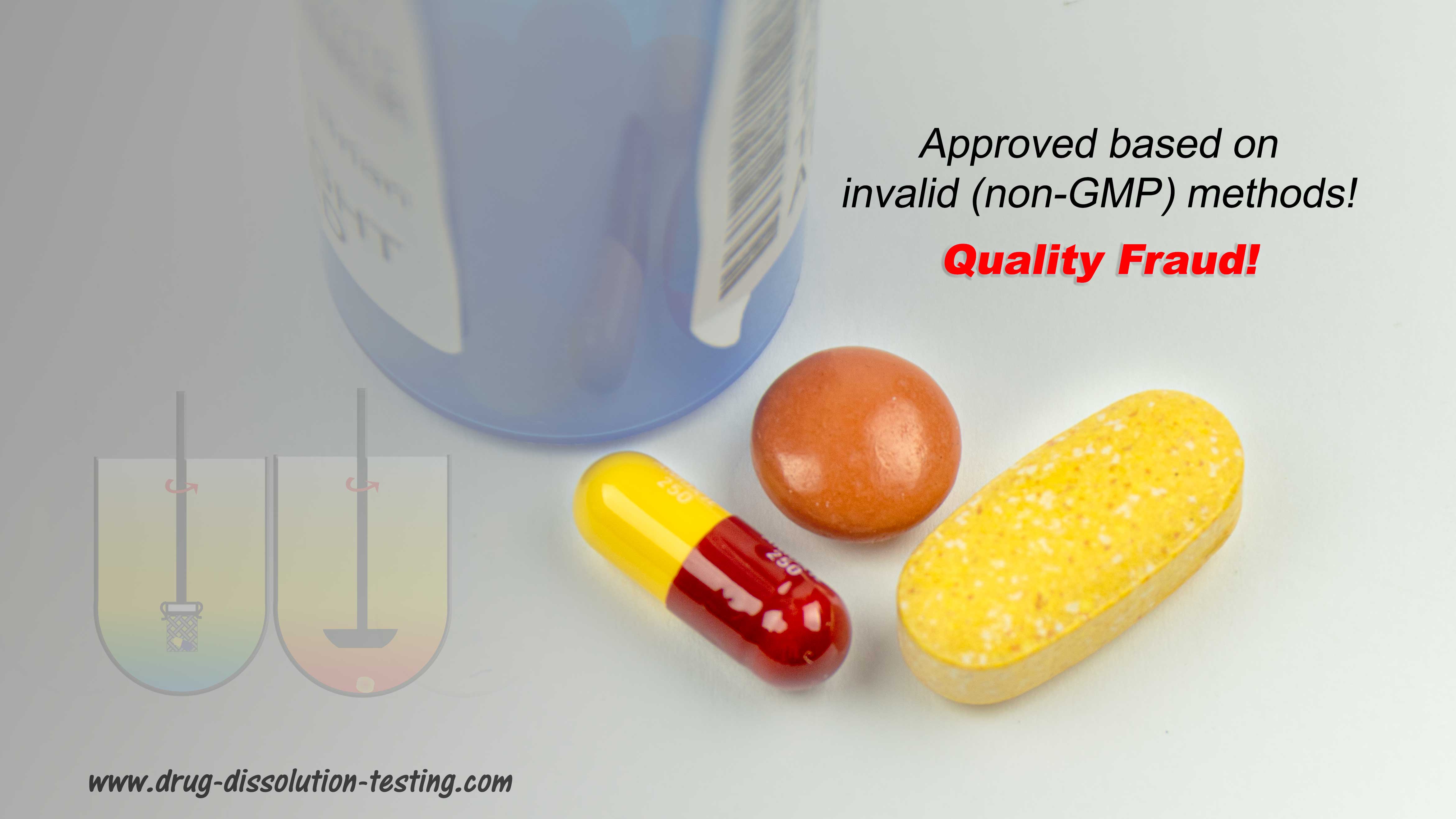
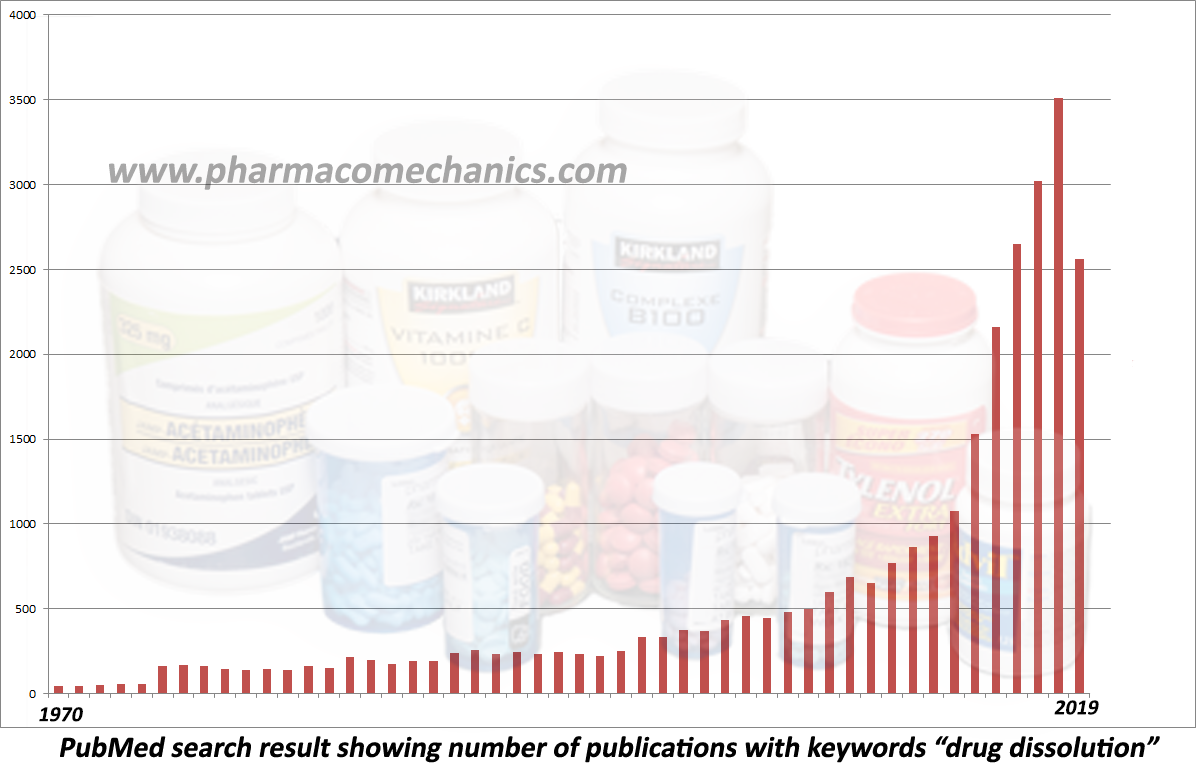
… provides in-depth discussion on establishing and monitoring the quality of pharmaceutical products in particular tablets and capsules. I hope you will find the chapter useful.
Chapter title: “Quality” of pharmaceutical products for human use – underlying concepts and required practices, published in
Drug Delivery Trends: Expectations And Realities Of Multifunctional Drug Delivery Systems Volume 3 Edited by Ranjita Shegokar, PhD., Available from Amazon (March 18, 2020), link.