A couple of days ago, I read two recent articles, one from the US FDA [1] and the other from the USP, defending and rationalizing regulatory and pharmacopeial practices and standards for assessing pharmaceutical products. These reports appear to respond to the concerns often expressed regarding deficiencies in regulatory product evaluations. This article provides an alternate view of the issues and suggests a solution for the authorities’ consideration. For the complete article, please follow the link (http://www.drug-dissolution-testing.com/?p=3198).
In the report from the US FDA, the emphasis is on justifying the current regulatory practices for adequately evaluating manufacturers or product manufacturing. It is claimed that the US FDA uses rigorous risk-based approaches in monitoring the plants (i.e., inspections) and evaluating pre-manufacturing submissions (i.e., NDA, ANDA). However, such claims may not be new, and the concerns persist, perhaps with increasing frequency and intensity. These concerns are due to mixing and confusing the two, i.e., manufacturing (or its quality) and the quality of the manufactured products. Authorities miss the difference and consider them the same thing. This misunderstanding confuses and is the root cause of the issues and concerns.
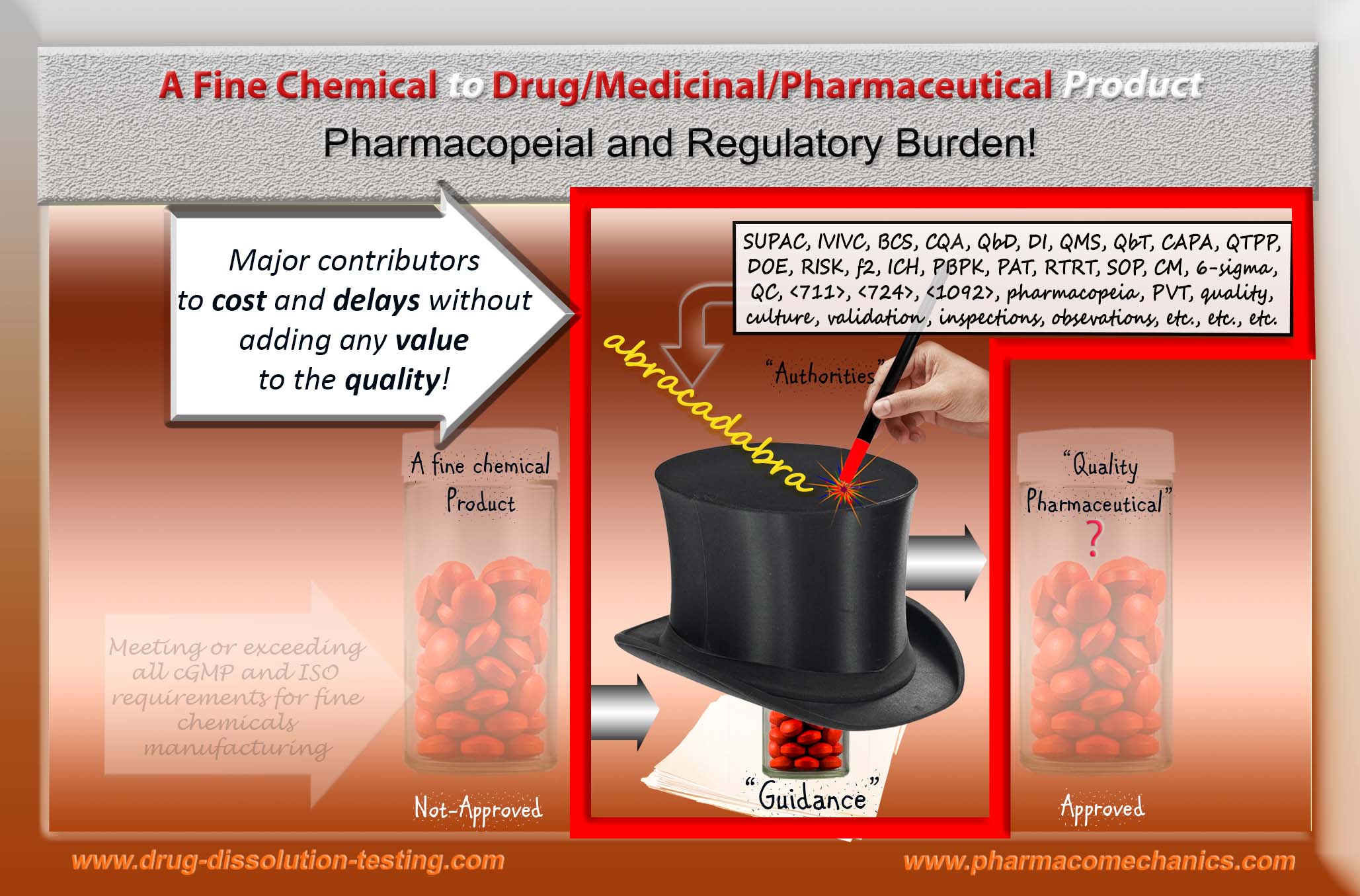
Public or patients need or require quality products, while the regulatory authorities have to ensure such. Unfortunately, there is no commonly accepted or official definition of a quality product; thus neither patient would get quality products, nor authorities can provide assurance. Authorities assume that if manufacturers meet the suggested regulatory processes and/or specifications (i.e., in compliance), then somehow the products manufactured would become of quality. On the other hand, if deviations from the processes and/or specifications are observed or perceived (which are often subjective), then the manufacturers would be labeled as non-compliant, and by extension, product quality is considered to be as substandard (or “adulterated”). Under the current regulatory system/requirements, it is really impossible to establish the quality of the products and their link to manufacturing.
It is important to note that, at present, product quality and manufacturing are based on in vivo (bioequivalence) and in vitro (drug dissolution) assessments. These techniques, as recommended by the authorities, are in fact scientifically in-valid and non-GMP compliant [3, 4]. It is, therefore, essential to recognize that regulatory assessments provide no scientifically valid support for establishing or monitoring the product quality, or lack of it, pre- or post-approval. Unfortunately, the manufacturers have been to bear the faults when guidance documents, standards, and specifications require attention and reconsideration.
The USP article rationalizes the approach of conducting performance of the dissolution testers used and required for establishing the quality of the products. This performance test is based on an arbitrarily selected product (which has no link to human use as a product) to establish validation of testers for the products for human use. In reality, claims made by the USP are scientifically invalid and simply false. USP promotes the use of these testers and tests for establishing the quality of products for human use, while these have never been tested or validated using any drug product for human use. If a company had made such a claim, it would have been disqualified and put out of business immediately (e.g., Theranos case [5]). However, USP and the US FDA promote these testers/tests for the quality assessment of the products. These apparatuses have clearly been shown to provide highly variable, unpredictable, and irrelevant dissolution results [6]. So much so that no one can determine appropriate and accurate dissolution characteristics of any product using USP apparatuses [7]. Therefore, authorities have to reconsider the use and requirements of the tests and testers.
To address the concerns and adequately assess product quality, authorities must define “product quality” with a measurable parameter and implement its use with scientifically qualified and validated tests and testers. Some suggestions in this regard are provided here [8]
[1] Statement from FDA Commissioner Scott Gottlieb, M.D., and Director of FDA’s Center for Drug Evaluation and Research Janet Woodcock, M.D., on the FDA’s continuing efforts to maintain its strong oversight of generic drug quality issues domestically and abroad (Link).
[2] The Critical Role of the USP Performance Verification Test in Dissolution Testing and Qualification of the Paddle Apparatus (Link]
[3] Time to rescind the regulatory requirements of bioequivalence evaluations and the current pharmacopeial drug dissolution practices as these do not provide quality assessment of pharmaceutical products (http://www.drug-dissolution-testing.com/?p=3065)
[4] “Regulatory (pharmaceutical) science” – lacks logic as well as science! (Link)
[5] Theranos (Link)
[6] Variability and unpredictability, everywhere! (Link)
[7] Note that no one can determine, or has determined, dissolution characteristics of any product using the currently suggested apparatuses and/or methods. It has all been an illusion! (http://www.drug-dissolution-testing.com/?p=1957]
[8] Seeking Metrics to Define Drug Quality (Link)
[9] Product Quality Metric (do not confuse it with drug/medicine quality) (Link).
[10] Universal Dissolution Test/Tester (Link)