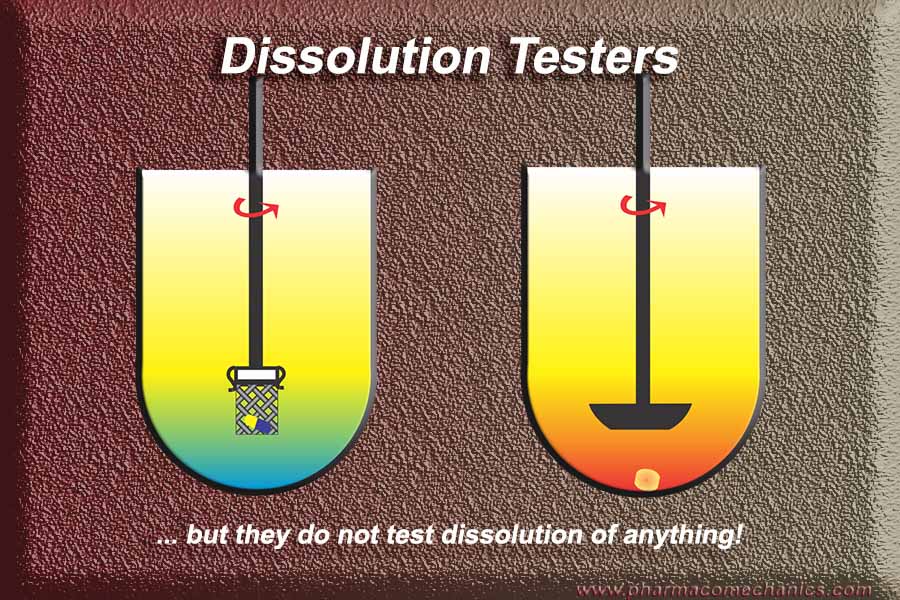
It is requiring drug dissolution testers, which do not (and cannot) determine dissolution characteristics of any product (tablet and capsule in particular). The amazing fact is that these are the only ones mostly recommended and accepted by the authorities, even sometimes as a substitute for clinical (bioequivalence) assessments. Why? How does it make sense – scientific or otherwise?
Some links for further details:
(1) Note that no one can determine, or has determined, dissolution characteristics of any product using the currently suggested apparatuses and/or methods. It has all been an illusion! (link)
(2) Bio-waivers! (link)
(3) Pharmacopeial Dissolution Testers (link)
