It is a well-established understanding that the drug/pharmaceutical industry is highly regulated. The elaborate regulations’ purpose and implementation are that the products manufactured should be of the highest quality standards possible. There are numerous sources of such regulations, most often country- or territory-specific such as US, Japan, Europe, etc., or some others harmonized such as from ICH. However, the most commonly referred or quoted are those from the US FDA, US Pharmacopeia, EMA, and ICH.
Regulatory authorities such as US FDA, Health Canada, EMA, and many others enforce such regulations and standards to ascertain that manufacturers and manufactured products are in compliance with regulations leading to the manufacturing of quality products. It is very important to note that a fundamental underlying assumption here is that if a product or process is in compliance then the product or process will be of quality. In general, such an underlying assumption is correct; however, this underlying assumption does not appear to be valid for the pharmaceutical industry.
In general, regulations and standards are based on, or derived from, scientific research following underlying scientific principles and theories. A common terminology that is used to describe these underlying scientific principles and theories is “validation,” i.e., the process, or processes, has been validated to provide quality products. Every step (small or large) is considered or broken further into smaller steps to validate the result (or product) should be of quality. For example, analytical techniques (such as chromatographic instruments and methods) may not be directly considered a manufacturing step but are critical in monitoring the outcome of manufacturing, thus requiring validation of their own.
From the scientific and regulatory compliance perspectives, validation of manufacturing processes, along with associated steps or components, is perhaps the most important and critical step and/or requirement. It is further critical to note that regulatory compliance requirements are, or at least should be, dependent on the well-established validation steps. It is not practical or useful to develop and/or implement compliance requirements and/or standards without having validation steps first.
So what does a validation step/process mean? In simple terms, if a claim is made, it must be substantiated based on scientific (mostly experimental) evidence. For example, suppose it is claimed that a product is of quality. In that case, it becomes mandatory first to state what quality means and then how this defined quality is established using scientific methods. The regulatory mandate is to evaluate if the quality is defined accurately, and then the methods and processes used are capable of measuring the quality of the products. In general, regulatory standards and requirements focus primarily on the outcome of manufacturing and may not be manufacturing itself, which for all practical purposes is secondary in the assessment of the outcome (products).
From a regulatory perspective, one has to deal with two aspects; (1) what is a “quality” product or what is “quality” of a product, and (2) how is it measured or established experimentally (scientifically). Unfortunately, however, it is disturbing that the quality of a pharmaceutical product has never been defined, particularly for regulatory assessment purposes. Therefore, it is not possible, at present, to know or establish whether a, or any, given product is of quality even if the regulatory authorities approve it. This situation needs to be addressed and corrected on an urgent basis.
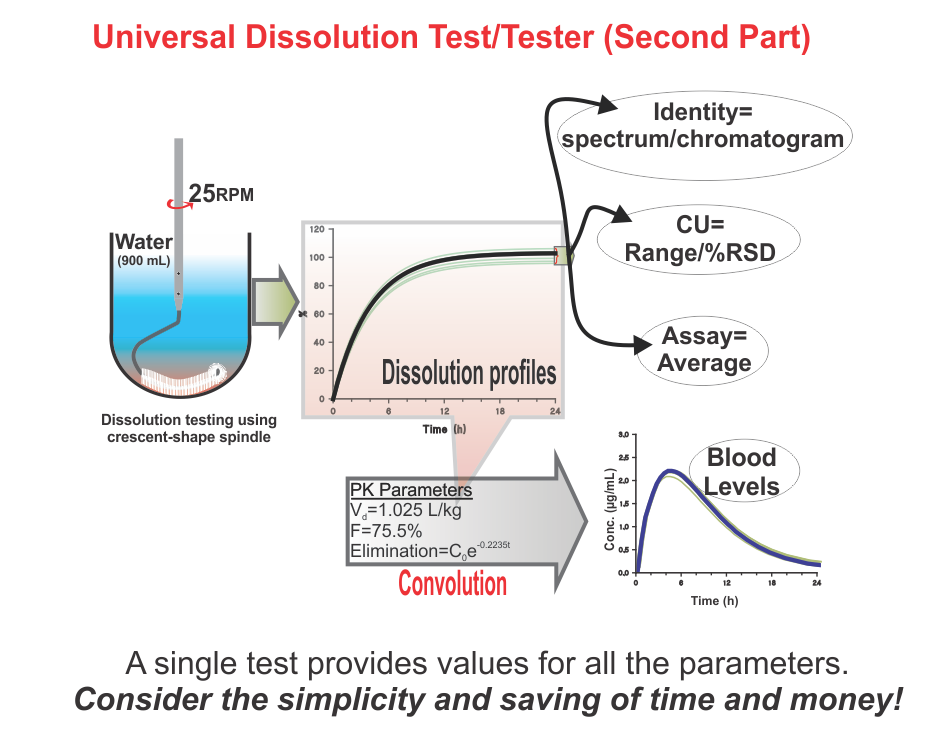
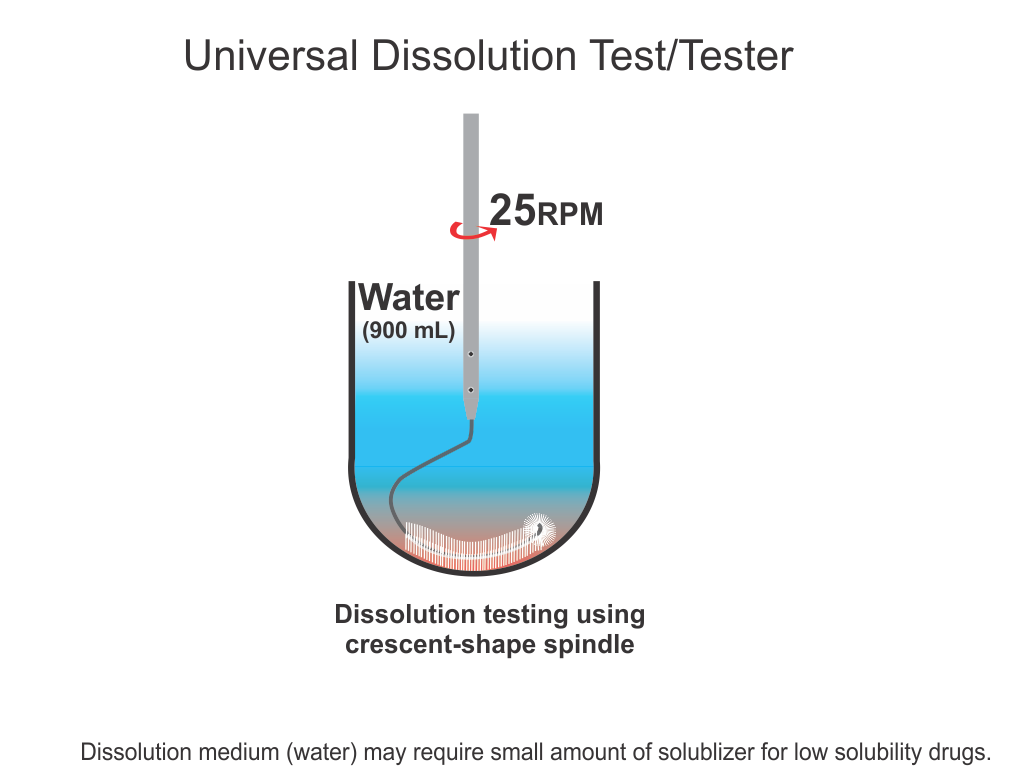
On the other hand, regulatory authorities suggest and enforce some traditional practices and standards, assuming that if manufacturers are in-compliance with such, the products would be considered of acceptable quality. Here again, there is a serious deficiency in suggesting compliance requirements. Often methods and techniques suggested, at least some, have never been validated for the claims made for them. For example, a technique known as drug dissolution testing, often mandatory for evaluating the quality of products, in particular oral such as tablet and capsule, has never been shown to provide any relevant characteristics of a product. There are so many stringent requirements for the required testers and testing methods without any scientific basis or reasoning. These are often extremely frustrating and resource-consuming exercises for the manufacturers and the regulatory authorities to meet compliance requirements for this test which has no link or contribution towards the quality assessment of the products. Recommendations and requirements for the use of non-validated and non-qualified testers and tests are serious violations of GMP requirements. Regulatory authorities should reconsider the current requirements of such testing on an urgent basis.
In short, as the quality of pharmaceutical products is an undefined parameter (metric), it is not possible that manufactured products can be assessed for quality. On the other hand, current regulatory requirements that manufacturers are to follow to comply with product approval are based on techniques and assumptions that lack scientific merit and/or validation of testers and methods, thus giving false hope or comfort about the quality of products.
The good news is that both of the issues mentioned above can be addressed with relative ease if a more logical and scientific approach/thinking would be pursued. I have extensively written about these issues and suggested possible solutions for addressing these issues by publishing in the scientific literature. Perhaps, the following articles would be useful as a start.
(1) Promoting quality standards for drug products: Scientifically speaking, please be systematic and logical! (link).
(2) Establishing safety, efficacy and quality of drugs and drug-products (tablet/capsule) – serious confusion! (link)
(3) The science of drug dissolution testing: Testers or apparatuses, experimental conditions and interpretation of results – A systematic approach for learning (link)