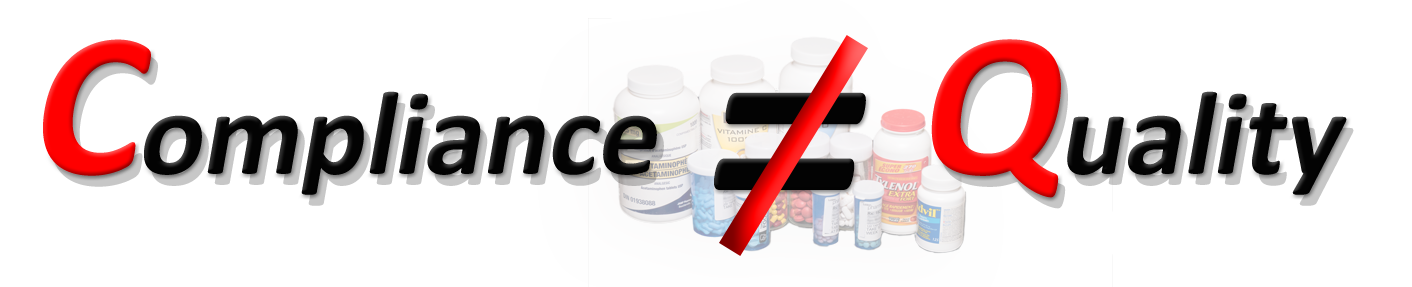
Quality has to be defined and measured independently. Currently, as a definition of the quality of a (pharmaceutical) product, such as a tablet/capsule, is unavailable; thus, it cannot be measured and/or established (link).
Consider attending the upcoming seminar to learn how the quality of a pharmaceutical product can easily be defined and then how it can be measured efficiently and scientifically (link).
Necessary knowledge of physiology, pharmacokinetics, pharmaceutics, or modeling/simulation can be easily acquired or learned. In addition, scientifically valid dissolution results can be easily obtained and transferred to plasma drug levels without involving the in vitro-in vivo correlation (IVIVC) step.
Focusing on defining quality and then establishing it by measuring its parameter will lead to simple, efficient and cost effective product development and manufacturing practices, significantly reducing regulatory burden.
