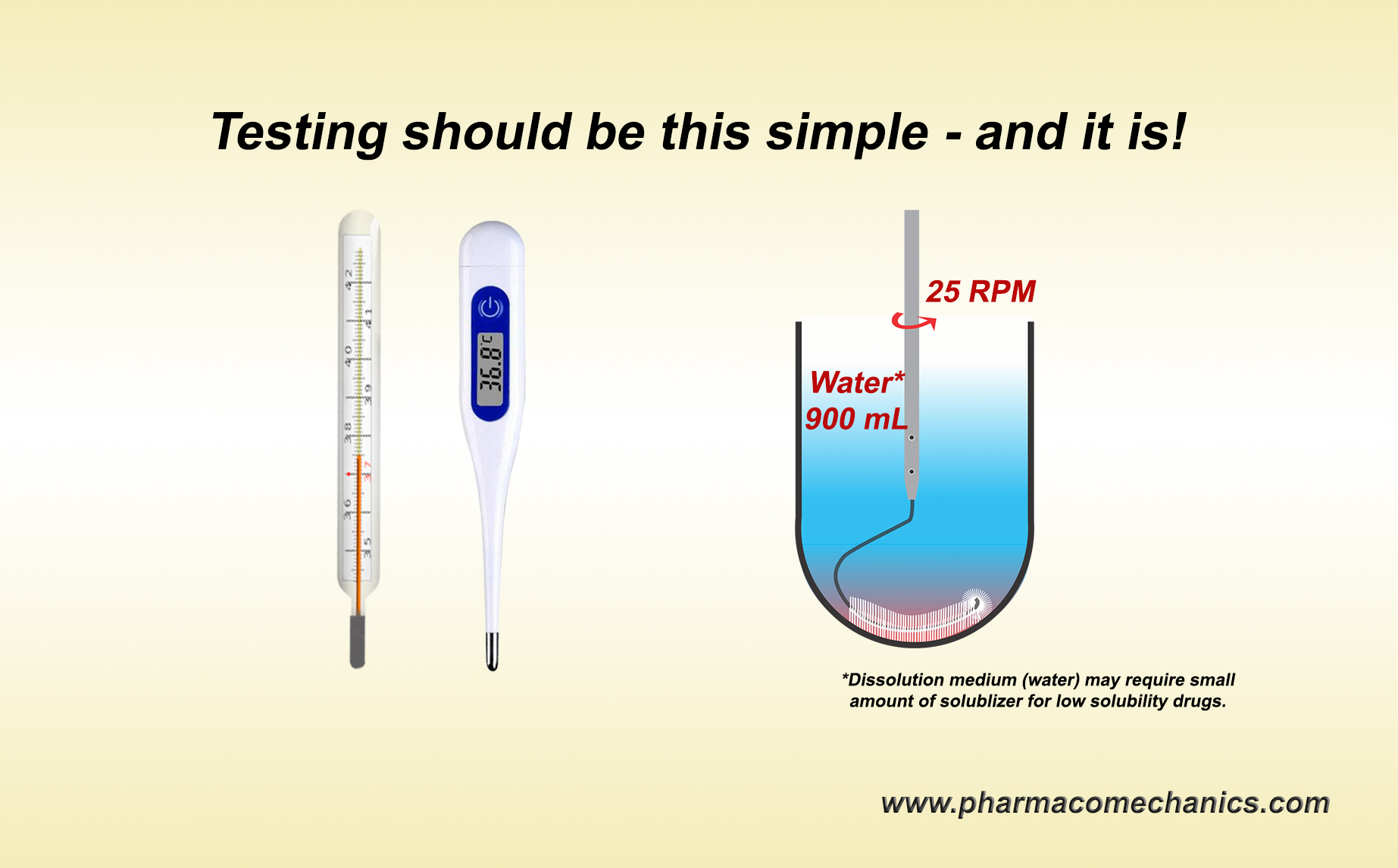
A discriminatory test, in general, is a test or procedure which would measure a given parameter and would be able to differentiate/discriminate between tested objects where the differences exist. A common and simple example of such a procedure or routine is monitoring body temperature using a thermometer. A thermometer is usually placed in the mouth, and after a bit of waiting, the thermometer provides a body temperature reading. Thermometers are pre-standardized/calibrated against reference standards (temperatures). There is no thermometer or monitoring procedure developments, no discriminatory test/procedure development, no “life-cycle” variations in procedures, and no subject-dependent (gender, age, race, body weight/type) procedures. Note that a thermometer does not even tell if the person has a fever. It will only tell body temperature, which is interpreted as whether the person has a fever. Point being a pre-standardized/calibrated thermometer by default becomes a discriminatory test or tester. This is how life/science works.
Similarly, a pre-standardized/calibrated dissolution test/tester must provide a product’s drug dissolution/release characteristic (tablet/capsule). Placing a product into a vessel equipped with a stirrer with pre-set rpm containing pre-defined solvent, its volume, and temperature is the dissolution test/tester. Determining drug release after a pre-defined time would provide the answer, i.e., drug dissolution characteristics of the product. Period! There should be no need for dissolution methods development of any type at the product/formulation development and/or evaluation stages.
Why do we have or need dissolution method development practices, including discriminatory methods – pure ignorance and incompetency of the subject? This is simply nonsense and fake-science! Be clear that no one is currently determining the dissolution characteristics of any product, hence the quality for which this test is conducted and promoted.
Seek sensible and scientifically valid solutions! (1, 2, 3, 4)
