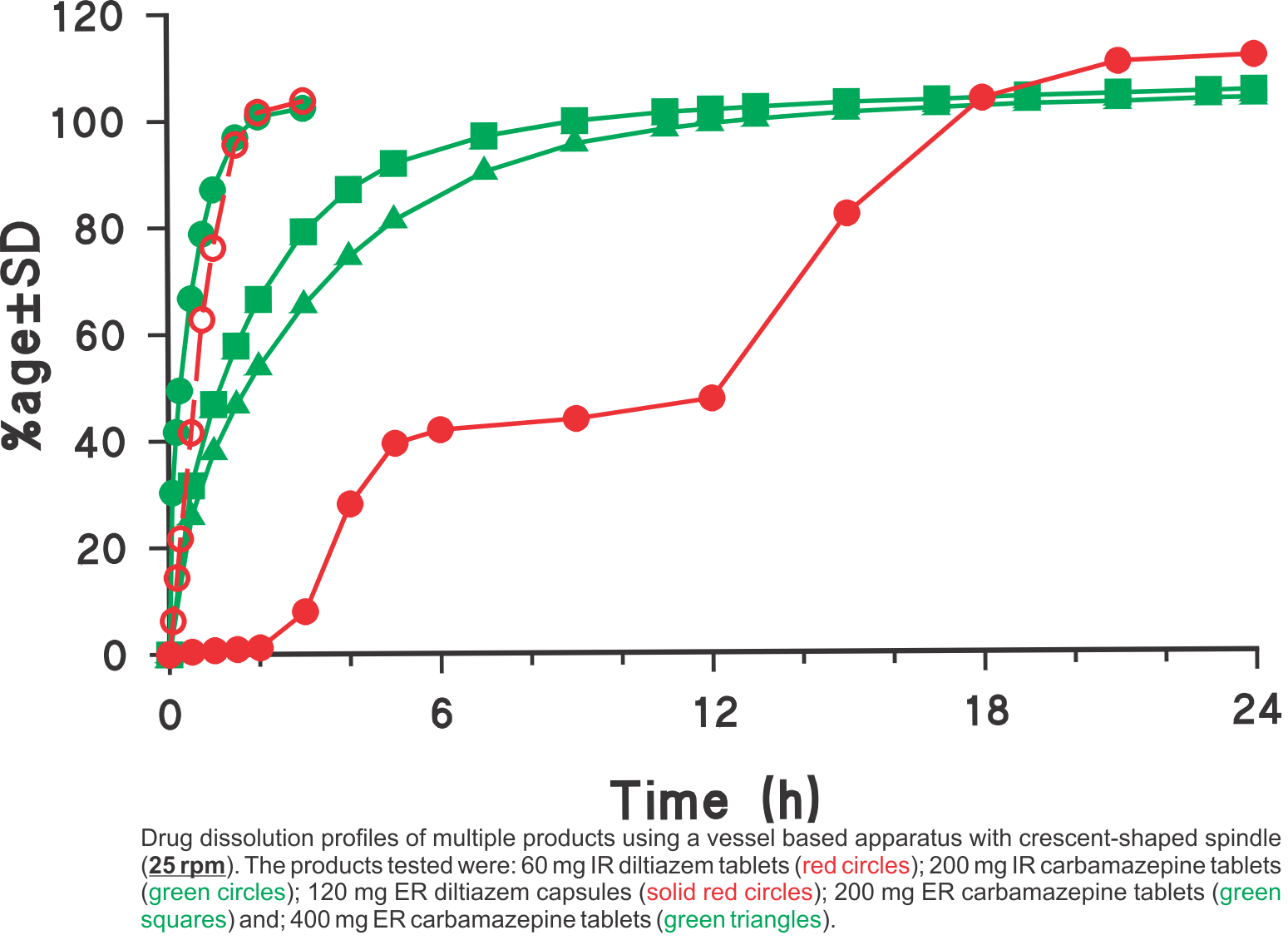
Dissolution profiles were generated using the USP vessel apparatus with crescent-shaped spindles set at a rotation speed of 25 rpm in all cases. The media used was 900 mL water for diltiazem and 900 mL water containing 0.5% sodium lauryl suphate (SLS) for carbamazepine products, respectively. The SLS was added to provide the needed sink condition. Using the suggested experimental conditions, all one has to do is to provide an appropriate dissolution medium (water with or without a solubilising agent e.g. SLS) so that the expected amount of drug is soluble in the medium. This single method/approach was employed for analyzing different types of products: tablets, capsules, IR and ER products having high water solubility (diltiazem) and low solubility (carbamazepine). As these experimental conditions are commonly used and simple, the method may easily be transferred to a QC test along with bio-relevant support of the testing environment. For further explanation and discussion on this topic please refer to the publication (link).
