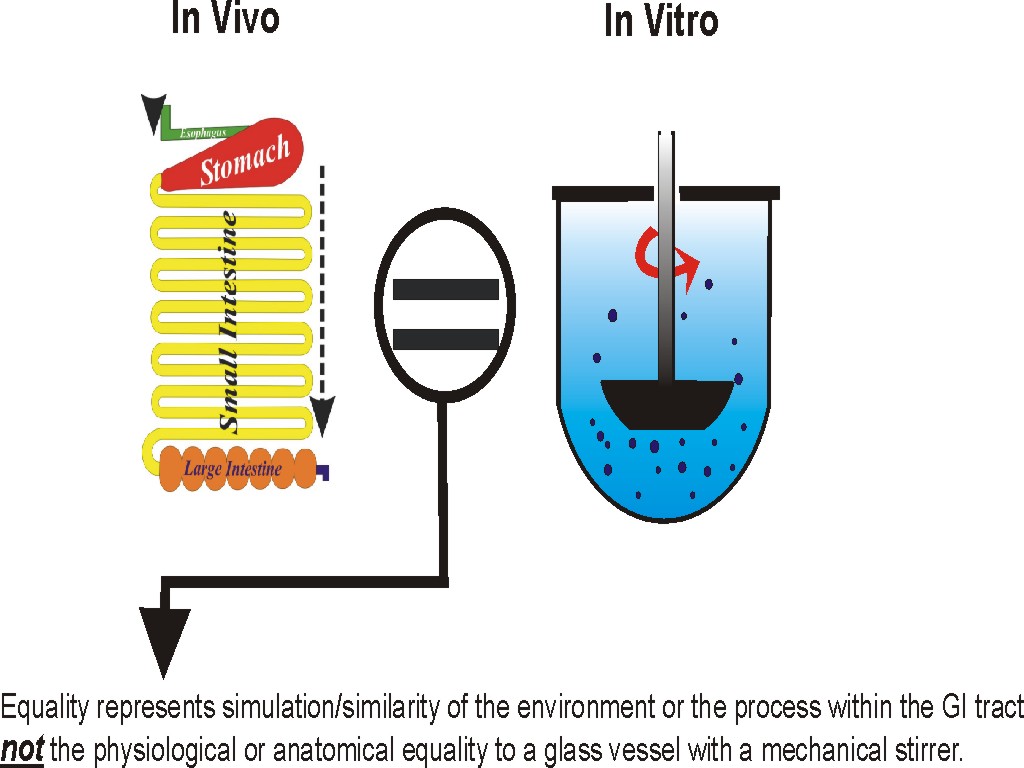
Drug dissolution tests are to be conducted using physiologically relevant experimental conditions. The physiologically relevant conditions do not imply that one needs to duplicate or simulate the anatomy or physiology of the GI tract in vitro, but that the environment or the process which facilitates the product/drug dissolution in vivo.
For example, for dissolution testing, one is required to maintain a temperature of 37 °C. However, it is not necessary or required that this temperature be attained or maintained in vitro by biochemical or enzymatic means as it occurs in vivo. The in vitro temperature can be attained and maintained by any means such as using a water bath, heating coils or plates, etc. The critical aspect is that the temperature has to be 37 °C.
Similarly, for in vitro dissolution purposes, one would require a container or vessel (reflecting intestinal space), water or aqueous buffer (reflecting liquid in the intestine), and a stirrer (reflecting churning within GI tract). These parameters should be chosen in such a way that they may or may not look or feel similar to the physiology/anatomy, but their effects have to be similar to the in vivo effects. The more one deviates from producing the in vivo effects in vitro. The less reliable and useful the drug dissolution test will become and vice versa. It is to be noted that, as described in the literature extensively, the currently used paddle and basket apparatus lacks in providing mixing or stirring (the equivalent of churning in vivo). Therefore, dissolution results obtained using these apparatuses may be of limited use or value.
Furthermore, as the physiological environment or process remains the same or constant from product to product, i.e., product independent, the in vitro experimental conditions should remain product independent. If dissolution testing requires changing of experimental conditions from product to product (e.g. IR vs ER), then that testing procedure needs to be reconsidered, as the in vitro (dissolution) procedure may not be valid.
