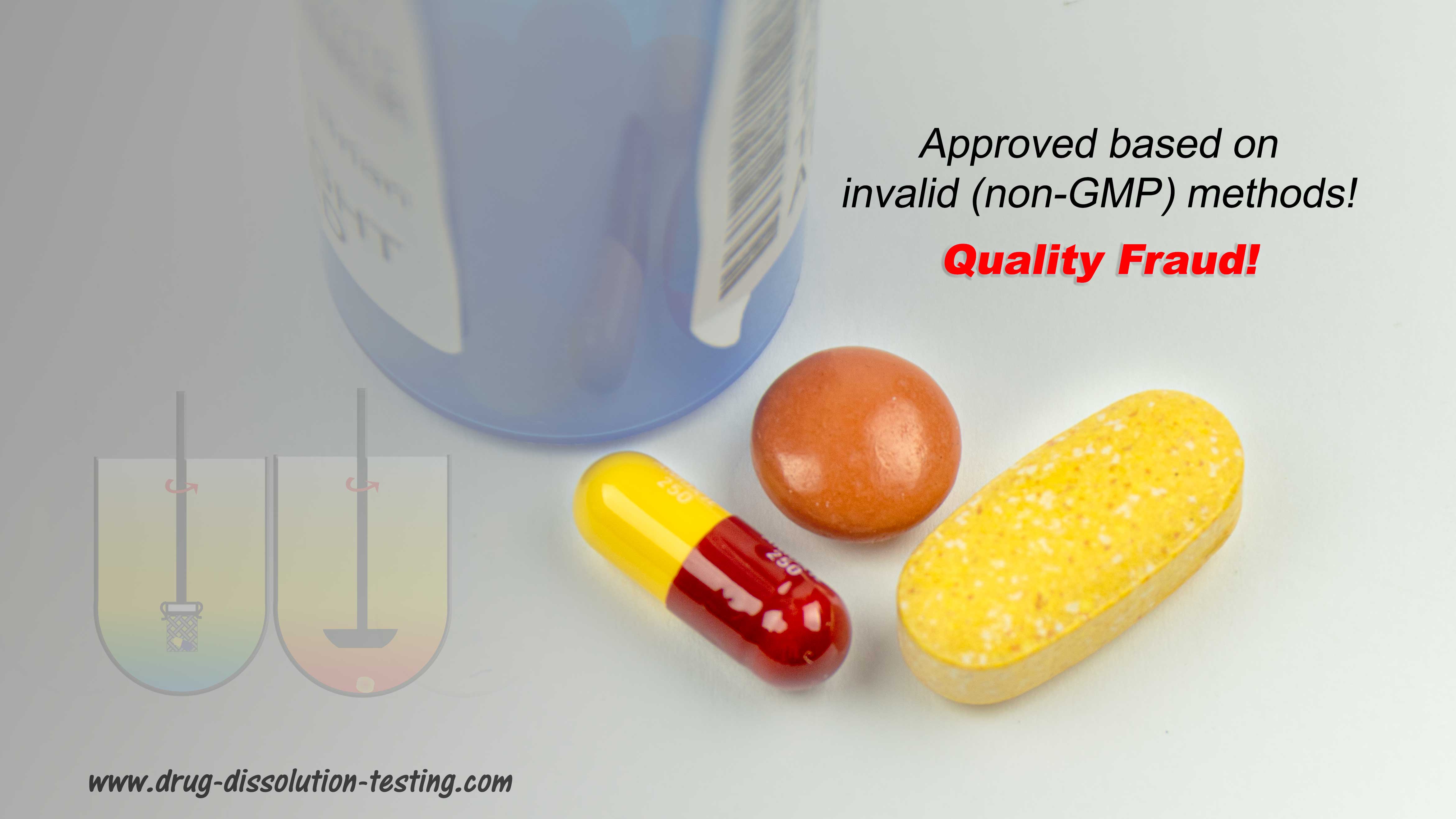
One cannot avoid being part of it. It is the regulatory agencies’ (e.g., FDA) and pharmacopeias’ (such as USP) practices and requirements to use the non-validated/non-qualified (hence non-GMP) testers and tests causing the fraud. Any claim concerning the quality of the products, in particular tablets and capsules for brand-name and generic products, must be false.
Blaming and punishing the industry for product quality and manufacturing are not relevant or valid and would not help. Instead, the authorities and pharmacopeias need education, advice, and help in defining quality and setting its standards and specifications (1, 2).
I will be happy to explain the issues with quality assessments in detail and can provide solutions to address them (link).
