USP diltiazem (ER) monograph describes 15 sets of tolerances or, by definition, “the permitted variation in the results (link)”; four sets for 12-hour and eleven sets for 24-hours products. Figures 1 and 2 present these tolerances in a profile format by using the average values of the ranges described. The first and last tolerances, where ranges are often described as NMT (not more than) or NLT (not less than) respectively, averages are calculated using zero and NMT values and NLT values and 110 (highest expected percent dissolved from the CU requirement).
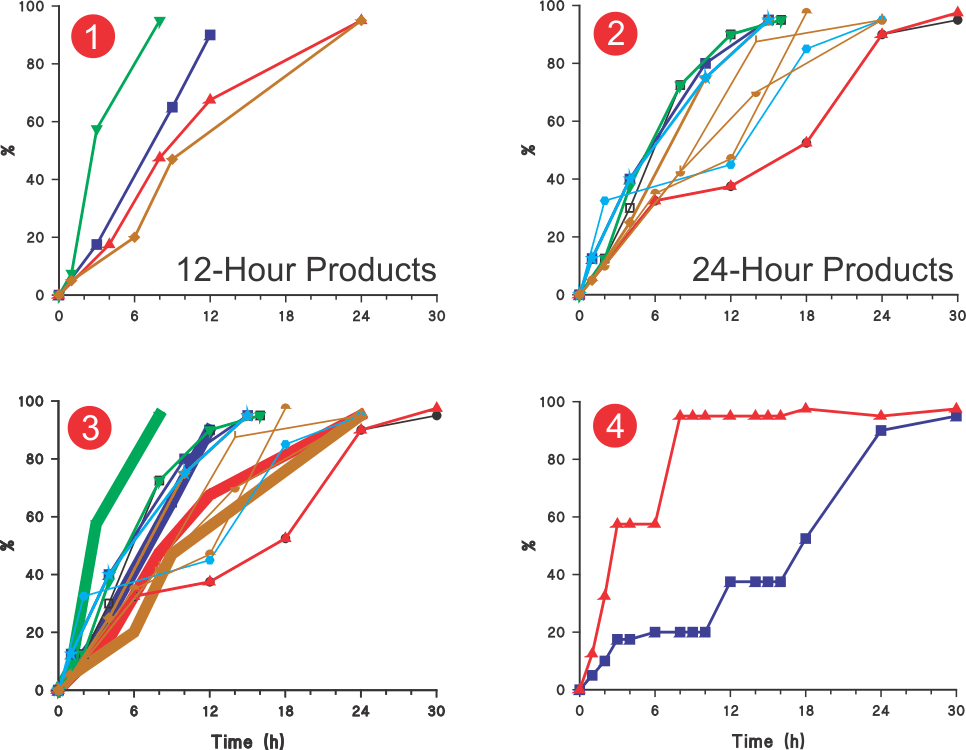
In short, these profiles represent acceptable variation in dissolution characteristics for presumably good quality (without a clinical concern) diltiazem products that are permissible for sale. In addition, although the monograph describes the tolerances for 12- and 24-h products separately, in most cases, these tolerances appear to overlap, as shown in Figure 3. Therefore, it would be safe to assume that, from the pharmacopeial perspective (in vitro testing), both of these types of products will show similar dissolution characteristics!
In most cases, tolerances are based on separate dissolution test conditions. Therefore, it is impossible to ascertain whether the dissolution characteristics reflect a product characteristic or because of the experimental conditions used. Therefore, it may not be possible to establish the quality of the product.
On the other hand, if such variations in drug dissolution (release) results are acceptable, the tolerances can be simplified by representing these only with one set of tolerances (upper and lower limit profiles) as shown in Figure 4. This would avoid the current complex and resource-intensive practices of setting individual tolerances with no apparent advantage. In addition, it will also help in establishing underlying expected variability in dissolution results.
