Product development stage: What this really means in simple terminology is the stage where a product (formulation + manufacturing process) is developed to show that it can release (dissolution) the drug and provide desired drug levels in humans. The drug release characteristics of a product are usually established based on human studies, which are commonly known as bioavailability/bioequivalence (BA/BE) studies. However, one requires a simpler in vitro method to screen test products (especially multiple combinations of formulations) to select some (usually one or two) for BA/BE studies.
Drug dissolution test: This is the in vitro test used for this purpose, i.e., to evaluate potential release characteristics of different products (or formulations). It is, therefore, very important to note that a formulator must have access to a dissolution method that can reflect potential in vivo drug release (dissolution) characteristics in humans at this stage. This method must already be developed and validated using other well-characterized product(s) for human use. In the literature, it is often described that a specific dissolution method is developed at this stage for the particular test drug/product. However, such a practice is scientifically invalid, as a method can only be developed using a product with well-established dissolution characteristics. At the product development stage, a dissolution test is applied, not developed. This is an essential concept, often overlooked, and should be kept in mind.
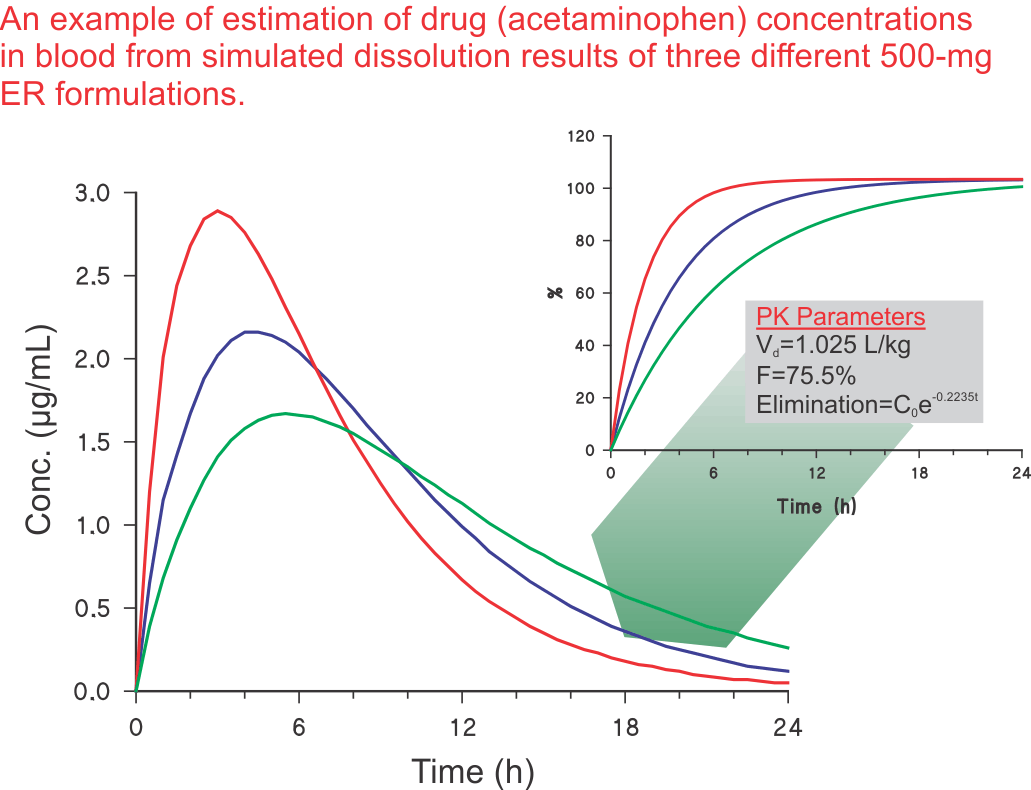
The next step: Preparing a variety of products (or formulations) and obtaining in vitro dissolution characteristics of these products would not be valuable or useful until it is shown what type of drug levels these products/dissolution results will provide in humans. It is important to note that obtaining similar or different dissolution characteristics for different formulations is usually of limited use. One has to demonstrate what would be the expected drug levels in humans from the test formulations. As stated above, the exercise of product development is to develop a product to achieve desired drug levels in humans. Therefore, there must be a technique to convert these dissolution results to potential drug levels in humans. Such a technique, known as convolution, is described in the literature (link), which may transfer dissolution results to blood levels in humans.
For an illustration, an example is provided to reflect the estimation of acetaminophen blood levels from dissolution results for a 500-mg extended-release product (see Figure). The methodology of convolution is described in detail in one of the publications (link).
Once a desired drug’s levels profile is achieved, the formulator may use the product for an in vivo BA/BE study to finalize the development.
If one follows the approach described here, it will provide a powerful screening method for selecting products for BA/BE testing and significantly simplify the product development stage.
