It is a very well-established fact that the USP apparatus PVT (Performance Verification Test) using prednisone tablets faces significant criticism regarding the lack of its relevance to the performance of the apparatuses. This criticism originates from the unexpected/unpredicted failures of the PVTs, by providing (dissolution) results outside the expected ranges, also called suitability ranges. Although not generally recognized, the main reason for such failures is that expected ranges are set tighter than needed to reflect the test’s true (high) variability. Therefore, in reality, when a PVT fails, it does not reflect a substandard apparatus or the testing but is a reflection of the actual/true variability of the testing. Often suggestions are given for addressing this situation by adjusting apparatus/testing parameters. However, a common view in the scientific community is that repeating the test, single or multiple times, often provides the desired outcome. Therefore, the current practice of PVT has become an exercise of obtaining dissolution results within an expected range rather than evaluating the performance of the apparatus/test.
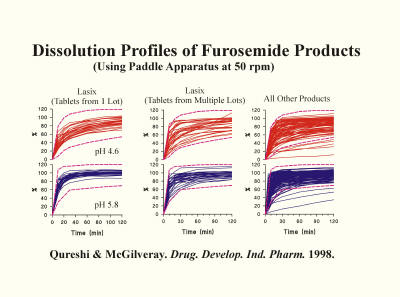
In addressing the potential cause of this high variability and unpredictability, a common focus has been the quality of the PVT (prednisone) tablets. However, if this had been true, the issue could have been resolved by using different tablets of the same or different active ingredients. In addition, there are many studies described in the literature using many other well-established products approved for human use, which demonstrate similar high variability in dissolution results. As an example, the figure below shows the dissolution characteristics of furosemide tablet products analyzed in different laboratories. The results show similar, if not worse, variability than of prednisone (PVT) tablets. Then, why is the high variability aspect often described with respect to prednisone (PVT) and not for other products? The reason is that prednisone (PVT) tablets are analyzed in multiple labs, thus higher visibility of the problem (variability). On the other hand, other products are usually tested internally, within the lab, or in few labs. Thus the problem is less visible. However, variability and unpredictability of results in both cases, PVT or products testing, is exactly the same.
The obvious logical conclusion from such observations would be that if the problem is not because of the tablets, the technique could potentially be the reason. However, studies are now reported in the literature, based on laboratory experiments and computer simulation exercises, which clearly demonstrate that the currently used apparatuses (Paddle/Basket) should indeed provide highly variable and unpredictable results. This variability and unpredictability results come from the poor flow dynamics within dissolution vessels, resulting in highly variable and unpredictable product-medium interactions. Therefore, it is a problem of the apparatuses/technique and not that of the products or PVT tablets, in particular.
Obviously, as the technique/apparatuses are the prime suspects of the high variability and unpredictability problem, then all results obtained using these apparatuses will become suspect, potentially undermining the quality of the products and adding cost to the product development and evaluation.
