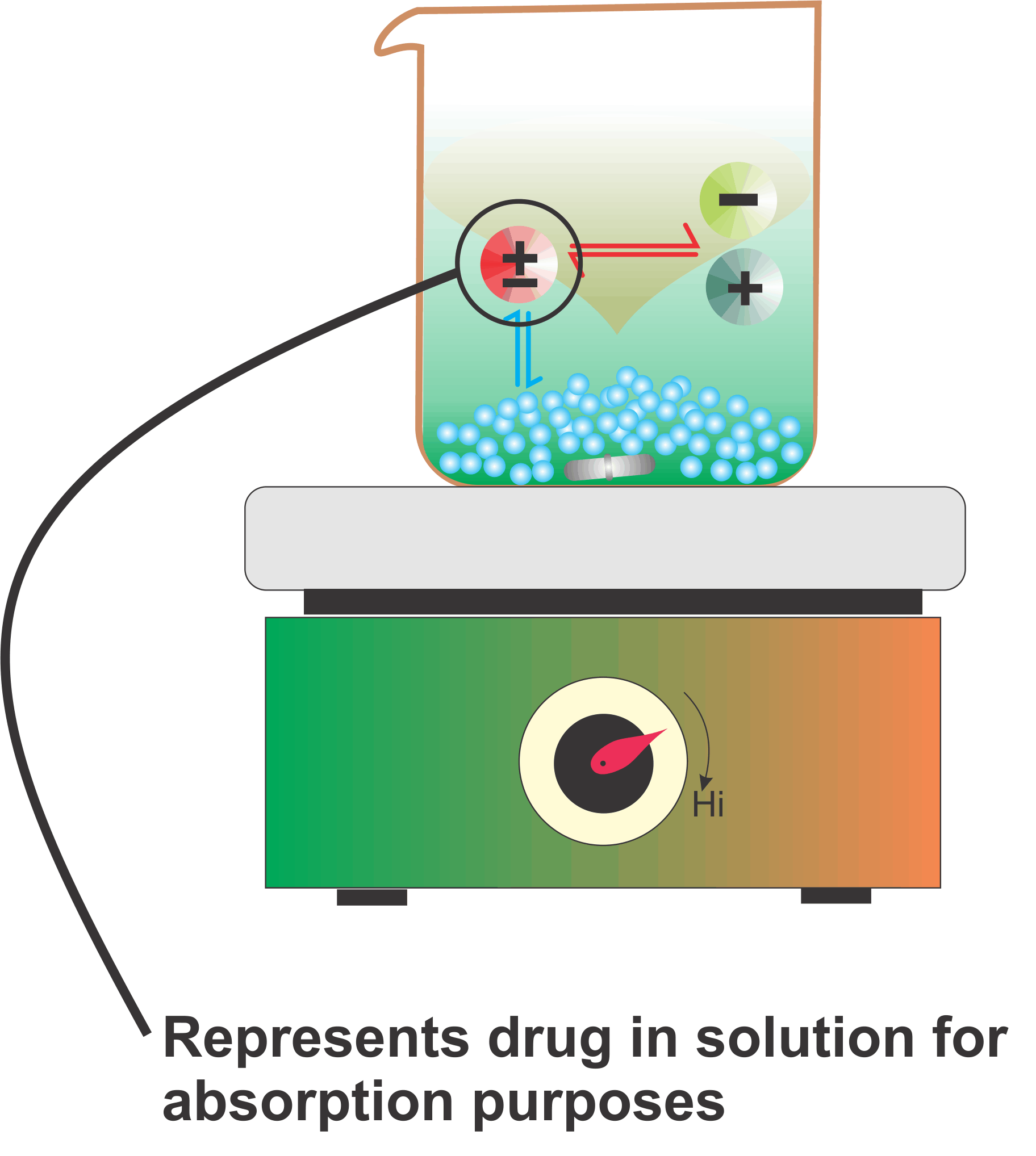
The Figure is a simple schematic representation for explaining the solubility of drugs for absorption purposes in the human GI tract for drugs that are weak acids or bases. These drugs dissociate into ions in equilibrium with the undissociated molecules (drugs) in the solution.
The undissociated drugs get absorbed, which disturbs the equilibrium with the corresponding ions. To maintain the equilibrium, the drug moves from the solid to the dissolved (undissociated) form, which gets extracted/absorbed. This cycle continues until the entire drug gets absorbed. The important thing to note here is that for complete absorption, drugs are neither required to be highly soluble nor need a large volume of solvent. It is this continuous extraction/absorption step that makes drug absorption possible and efficient.
For further discussion on the topic, please see the following links: (1, 2, 3, 4)
