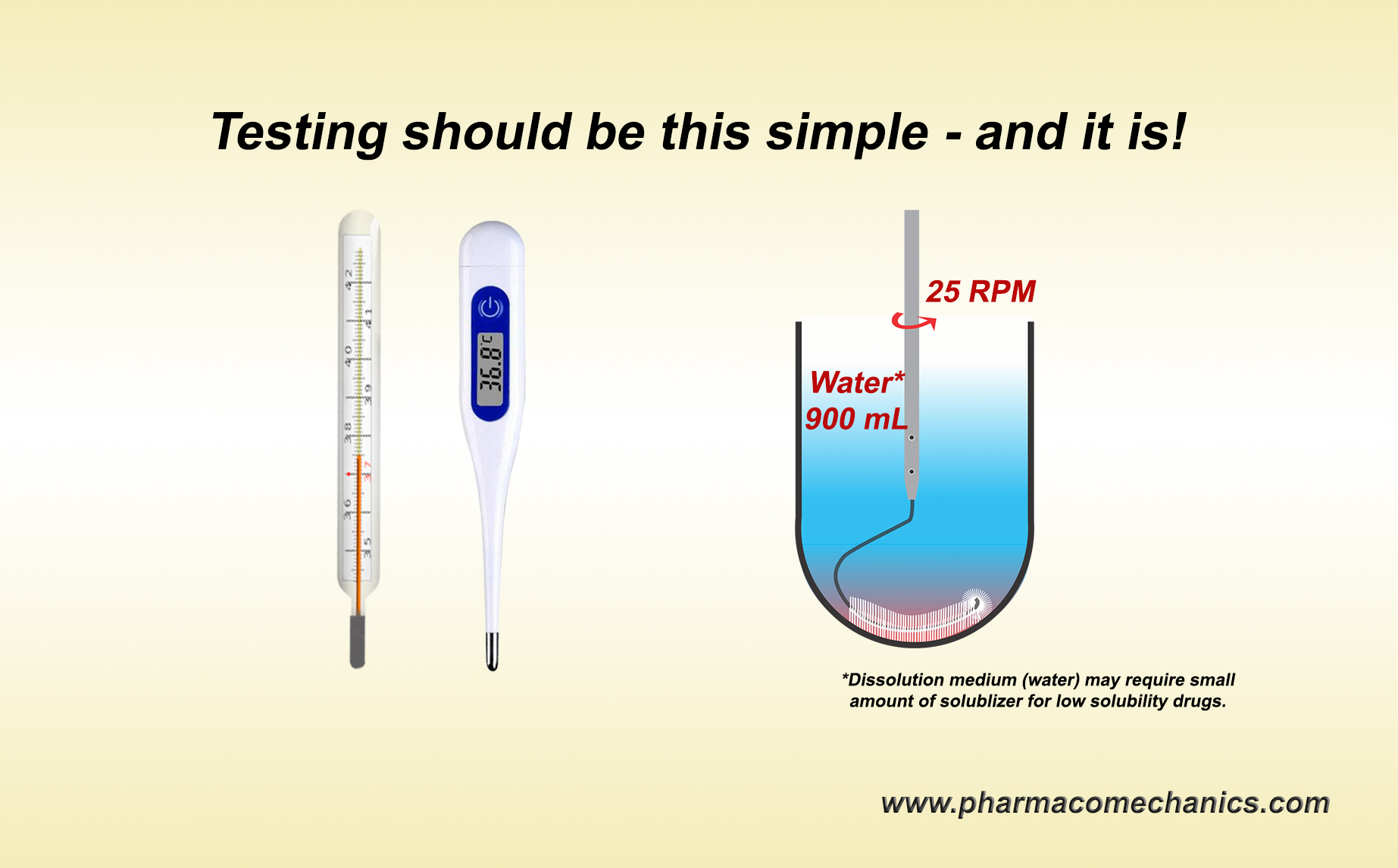
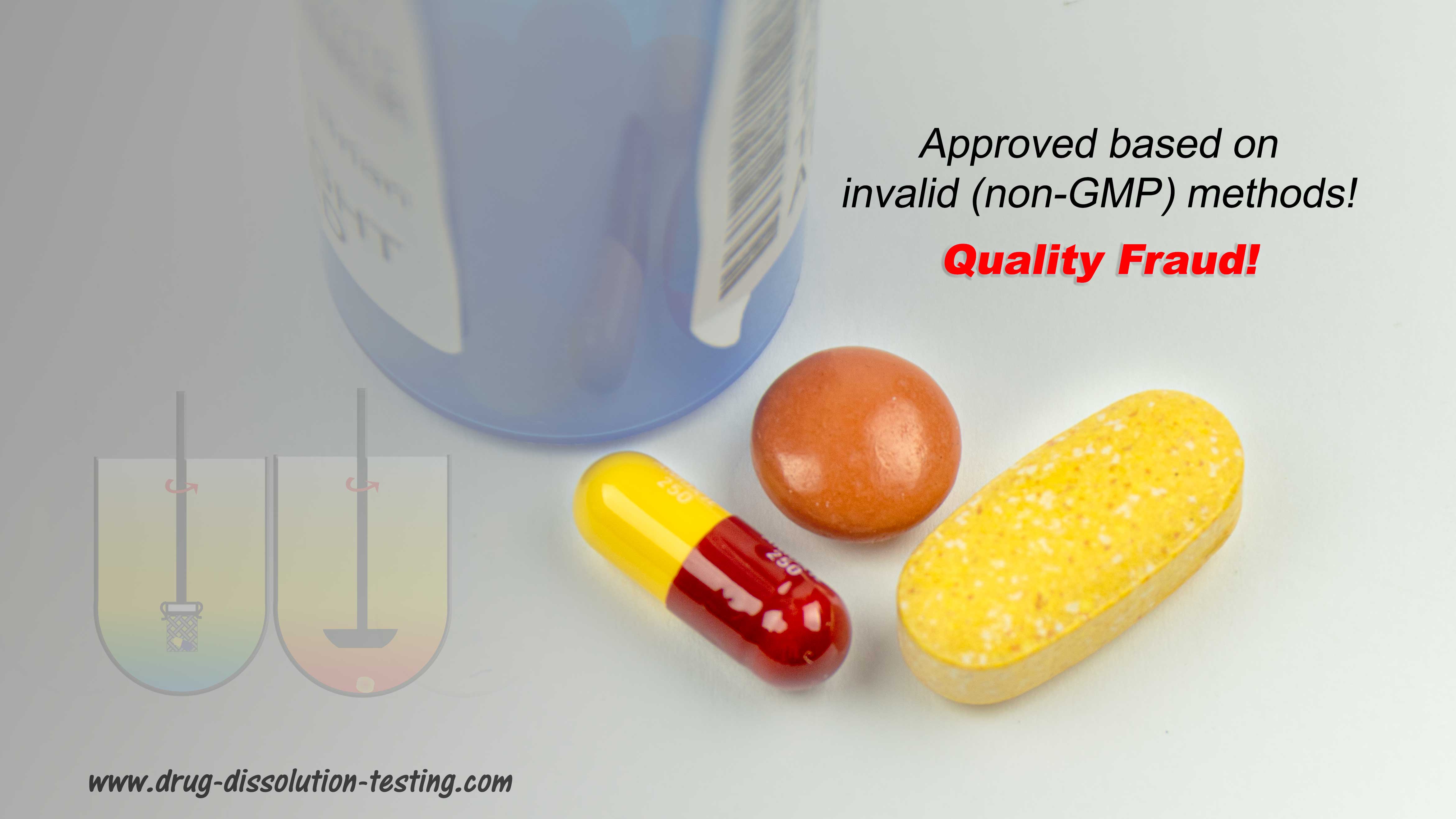
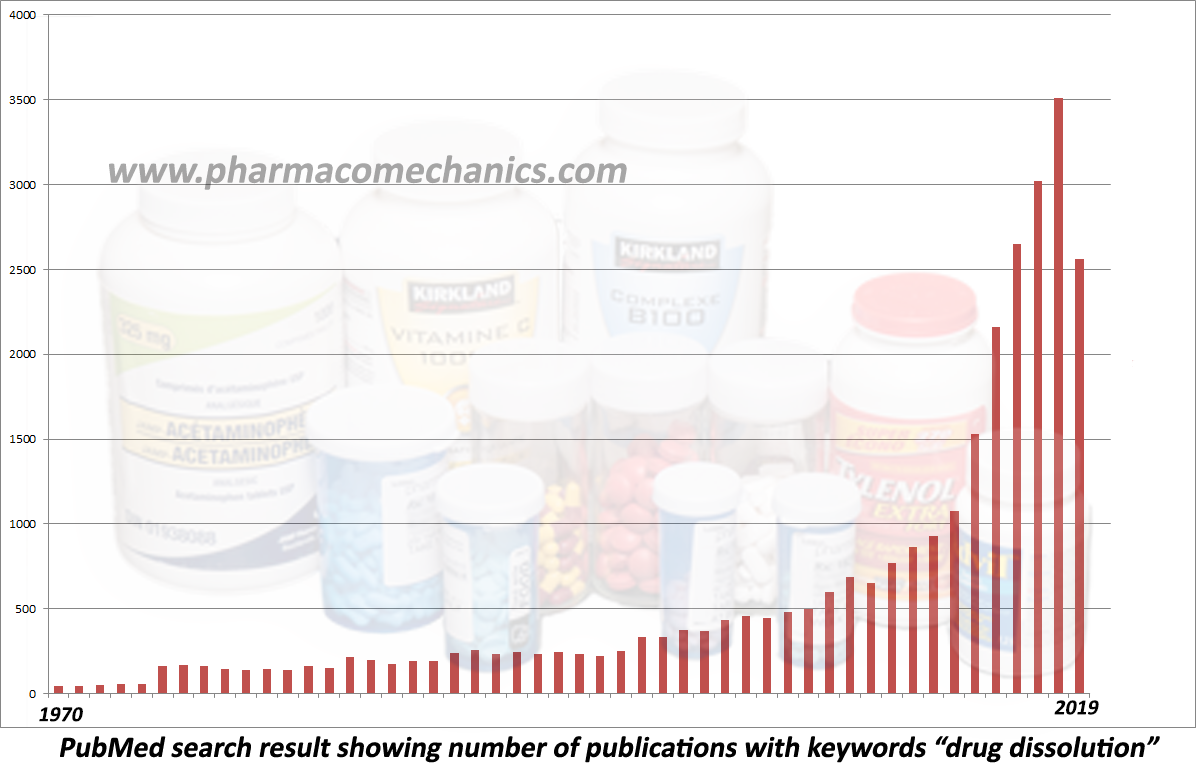
These are often visitors to manufacturing facilities under the titles of investigators, inspectors, auditors, consultants, experts, compliance officers, etc. (internal or external to the regulatory agencies). Logically and scientifically speaking, they provide no useful or valuable purpose for developing, manufacturing, and assessing pharmaceutical products such as tablets and capsules. They cause hindrances, interruptions, and exuberant costs to industry and by extension to consumers/patients. Their observations and conclusions are based on subjective and narrative discussion, not scientific, measurable, or quantifiable assessments. Their reports are often filled with fancy catch phrases or acronyms such as cGMP, validations, CSV, data integrity (DI), record-trailing, inappropriate SOPs, improper documentation, root cause analysis, and CAPA. These phrases are, in general, book/record keeping (admin/clerical) exercises mostly unrelated to the quality of the products and/or the manufacturing quality. Note that any deficiency or observation, unsupported with corresponding direct and/or validated evidence of the quality of products or their manufacturing (which often is the case), must be rejected as superficial or irrelevant. The industry should protect its employees’ expertise, academic credentials, and manufacturing competence from these “visitors,” who often lack the needed expertise. Seek help protecting your expertise and manufacturing quality products with scientific evidence, knowledge, and support. The following links to articles describing the basis of irrelevancy of often reported deficiencies.
(1) Biggest violators of GMP (link)
(2) Can Regulatory And Pharmacopeial Compliance Practices Establish Quality? (link)
(3) Consumers and patients must wait and suffer for the availability of quality pharmaceutical products such as tablets/capsules and their genuine and affordable prices. The reason may surprise you! (link)
(4) Making unsubstantiated and/or false claims by the regulatory authorities, including FDA, regarding the quality of pharmaceutical products (link)
(5) Pharmaceutical product manufacturing as per current regulatory requirements! (link)
(6) Promoting quality standards for drug products: Scientifically speaking, please be systematic and logical! (link)
(7) Quality assessment of pharmaceutical products – regulatory/pharmacopeial standards and methods require urgent attention! (link)
(8) Regulatory/pharmacopeial assessments of the quality of the pharmaceutical products – in the grip of falsehood and fraud! (link)
(9) Time to rescind the regulatory requirements of bioequivalence evaluations and the current pharmacopeial drug dissolution practices as these do not provide quality assessment of pharmaceutical products (link)
(10) Why are regulatory authorities, including pharmacopeias, allowing and promoting (through guidance documents and seminars/conferences) the use and sale of non-GMP-compliant drug dissolution testers? (link)