A few days ago, FDA responded, as a final response, to my citizen petition submitted on October 2, 2018, by denying the petition.
The response acknowledges the flaws in FDA requirements, as highlighted in the petition. However, unfortunately, the petition was denied based on irrelevant discussion and arguments on method development and validation, not on apparatuses/testers validation – the subject of the petition [link].
The petition stated that “… as per cGMP requirements every equipment, including apparatuses/testers, used for drug product manufacturing and evaluation must be validated [e.g. see 6-8] to demonstrate that the equipment or testers are qualified and validated for the intended use and purpose. However, the drug dissolution testers have never been validated for their intended use.“
In the response from the FDA, denying the petition after almost 4-years of wait or study, it is stated that “Method validation and verification encompasses the apparatus used in the method; the apparatus is not separately validated.” Therefore, confirming that apparatuses/testers are not validated, i.e., FDA (product quality) requirements are based on non-validated apparatus or testers. It infers that all methods developed using such testers and the results obtained have to be non-validated – null and void.
The 7-page FDA response covers the method validation explanation, which is separate from the apparatuses/tester validation and irrelevant to the petition’s requested action.
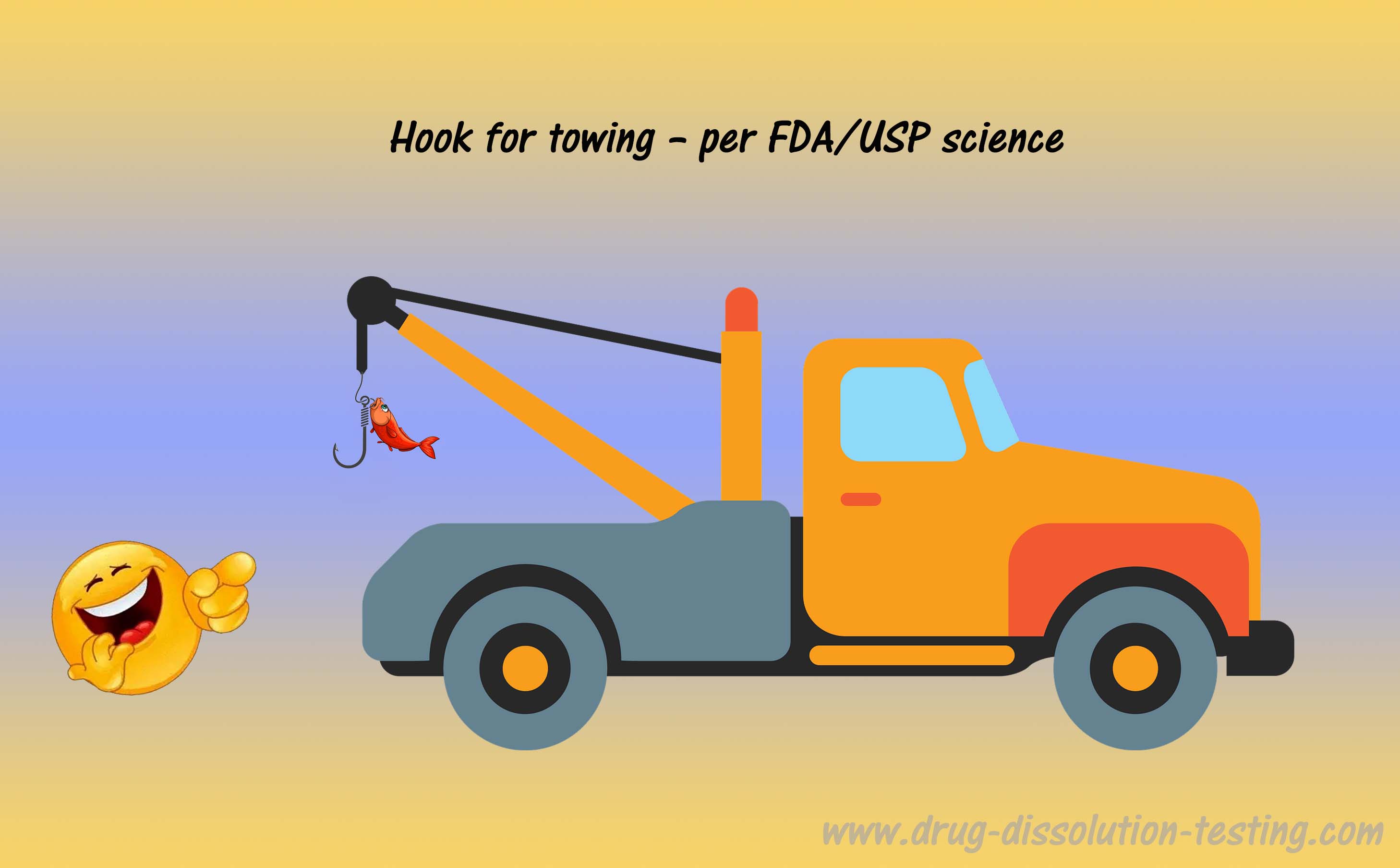
It is critically important to note that qualification (or “qualified tester”) represents only the physical appearance of the item, e.g., any fishing hook would qualify as a tow truck hook. However, validation establishes the relevance and usefulness of the item for the intended purpose. Therefore, a fishing hook may be qualified but not be considered validated for the intended use. The validation step can only be conducted under actual use conditions, where the fishing hook will fail to perform the towing.
Similarly, an FDA/USP dissolution tester may be qualified for dissolution testing, like a fishing hook for towing. However, the tester’s validation (relevancy and applicability) can only be established using an actual physical sample of a drug product for human use, which has never been done. So, in layman’s language, current dissolution testers are equivalent (“qualified”) to tow trucks with fishing hooks.
As noted above, cGMP (current Good Manufacturing Practices) requires the apparatuses and tester to be qualified and validated before use. Unfortunately, the FDA/USP dissolution testers have never been validated (as acknowledged by the FDA in the petition response). Therefore, they do not meet the cGMP requirements. Hence these testers must not be used or recommended for pharmaceutical product evaluation. Otherwise, it amounts to scientific fraud, as explained here.
So, to conclude, FDA requirements for assessing the quality of products using the drug dissolution technique are based on non-validated apparatuses and testers, not in line with FDA’s cGMP requirements. Their use is a clear example of stark incompetence and ignorance of the science of testing/testers and its validation at the FDA/USP. An independent third-party audit in this regard is urgently needed.
Such an independent third-party audit will also help in correcting the error made by regulatory authorities (CDC and FDA) in using non-validated (PCR and Rapid Antigen) tests for the COVID-19 virus (SARS-COV-2) assessments (see here).
