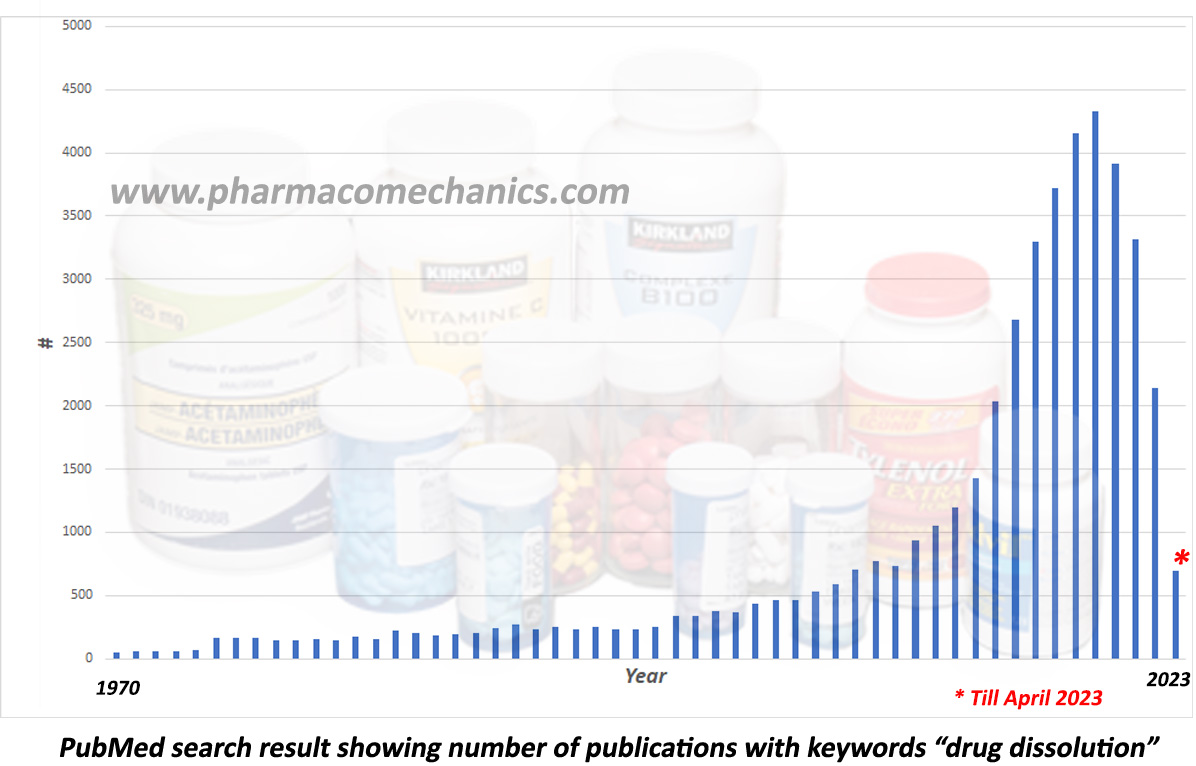
In 2020, I wrote an article (link) stating that drug dissolution testing is in decline. Checking it now (see the figure above) confirms that testing has declined significantly. For example, the reported number of dissolution-related publications is about half in 2022 compared to 2019 (the highest number reported). The trend appears to continue in 2023. This decline indicates hesitancy in using testing, most likely because of the irrelevancy and invalidity of drug dissolution results or data. Currently, dissolution testing is based on using non-validated testers. Not only should such testers be illegal to use, but any data obtained must be discarded as irrelevant.
While rejecting my Citizen Petition, FDA acknowledges (link) that these testers have not been independently validated. Therefore, technically and scientifically, these testers should not be part of any drug product evaluation.
There is a strong possibility that dissolution testers and their promoters will face the treatment as Theranos for using non-validated testers (link). When the time comes, the excuse that dissolution testers are only for compliance will be considered an inapt argument.
Dissolution testing is a critical step for product evaluation. However, it has to be conducted using testers that would provide efficient stirring and mixing and validated for the intended purpose, which is lacking. Therefore, people should start thinking about it before too late.
My Helpful Note provides help in addressing various issues and options in this regard.
