
A drug dissolution test is a test used to establish the quality of a pharmaceutical product, such as a tablet or capsule. This test is conducted because the drug (often called active ingredient) needs to be released from the product for its absorption into the blood to elicit its therapeutic effect.
The test is conducted in place of a clinical test/study called bioavailability or bioequivalence, a pharmacology/pharmacokinetic study type. The test is the backbone of quality assessment of all tablet and capsule products. It is a requirement of worldwide authorities, including the FDA, Health Canada, etc., and pharmacopeias such as USP, BP, EP, etc.
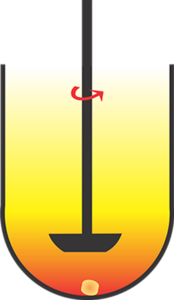
The test is straightforward: stirring a product (tablet and capsule) in a glass vessel containing water or aqueous buffer, usually 900 mL, maintained at 37C (body temperature). These test/experimental conditions simulate the GI tract environment, where the drug is expected to be released and absorbed.
If one is required to test the release property or the quality of a product, all one needs to do is drop a tablet/capsule, run the test, and monitor the drug release from the product by monitoring drug content in the vessel or solution.
So, what can be wrong here? Everything!
The test is not done like this. It is done by assuming (first) the expected drug release or quality and then adjusting the experimental conditions (dissolution medium, pH, stirrer, stirring speed, etc.) to show the desired release characteristics.
In layperson’s terms, it is like producing a piece of something (say food, a slab of butter) assumed to weigh 1 kg; rather than measuring using a standard weighing scale, the company develops a brand new scale to show the presumed weight.
Please do not laugh! This is how pharmaceutical scientists measure the drug dissolution characteristics of tablets/capsules, hence their quality. Hundreds of such drug dissolution tests/scales have been developed, asserting that they are based on science to assess the quality of tablet/capsule products. As of July 2024, 1800+ such scales/tests are described in the United States Pharmacopeia (USP), the standard-setting organization for pharmaceutical product quality (link).
It is important to note that no standard test/scale or reference table/capsule product is available.
To sound scientific, creating these drug dissolution scales is described as a drug dissolution test/method development: research institutions and industry spend millions of dollars on this (useless) exercise.
The US FDA provides documents for developing such scales under “Product-Specific Guidances for Generic Drug Development” (link).
Would you buy a product whose quality is established by testing against itself by adjusting the scale? Most likely not. However, it is like that in medicines, claiming that the products are of the highest quality, per US FDA and the USP assurance and approval.
As a physician and patient, you have thoroughly been fooled in the name of science – the fake/fraud science – and there is no assurance of the quality of these products. Only based on the authorities’ view and narrative – no science, no logic.
Please watch for claims of science in medicine; there are none!
For further reading:
A New Crescent-shaped Spindle for Drug Dissolution Testing—But Why a New Spindle? (link)
FDA acknowledges the use of non-validated drug dissolution testers – cGMP violation! (link)
Regulatory/pharmacopeial assessments of quality of the pharmaceutical products – in the grip of falsehood and fraud! (link)
